Indications/Uses
Treatment of symptoms of gastro-oesophageal reflux such as acid regurgitation, heartburn and indigestion, for example following meals or during pregnancy, and for symptoms of excess stomach acid (hyperacidity).
Dosage/Direction for Use
If symptoms do not improve after seven days, the clinical situation should be reviewed. Prolonged use should be avoided.
Liquid: Adults and Children 12 Years and Over: One to two sachets or doses (10-20 mL) after meals and at bedtime, up to four times per day.
Children under 12 Years: Should be given only on medical advice.
Elderly: No dose modifications are necessary for this age group.
Tablet: Adults and Children 12 Years and Over: Two to four tablets after meals and at bedtime, up to four times per day.
Children under 12 Years: Should be given only on medical advice.
Elderly: No dose modifications necessary for this age group.
Administration: Liquid: For oral administration.
Tablet: For oral administration after being thoroughly chewed.
Liquid: Adults and Children 12 Years and Over: One to two sachets or doses (10-20 mL) after meals and at bedtime, up to four times per day.
Children under 12 Years: Should be given only on medical advice.
Elderly: No dose modifications are necessary for this age group.
Tablet: Adults and Children 12 Years and Over: Two to four tablets after meals and at bedtime, up to four times per day.
Children under 12 Years: Should be given only on medical advice.
Elderly: No dose modifications necessary for this age group.
Administration: Liquid: For oral administration.
Tablet: For oral administration after being thoroughly chewed.
Overdosage
Symptoms are likely to be minor; some abdominal distension may be noticed.
Management: In the event of overdose, symptomatic treatment should be given.
Management: In the event of overdose, symptomatic treatment should be given.
Administration
Should be taken with food: Take after meals & at bedtime. Chew tab thoroughly before swallowing.
Contraindications
Hypersensitivity to sodium alginate, sodium bicarbonate, calcium carbonate or to any of the excipients listed in the product.
This product should not be used in patients with moderate or severe renal insufficiency.
This product should not be used in patients with moderate or severe renal insufficiency.
Special Precautions
Liquid: Each 10 mL dose has a sodium content of 127.25 mg (5.53 mmol). This should be taken into account when a highly restricted salt diet is recommended, e.g. in some cases of congestive cardiac failure and renal impairment.
Each 10 mL dose contains 130 mg (3.25 mmol) of calcium. Care needs to be taken in treating patients with hypercalcaemia, nephrocalcinosis and recurrent calcium containing renal calculi.
Treatment of children younger than 12 years of age is not generally recommended, except on medical advice.
If symptoms do not improve after seven days, the clinical situation should be reviewed. Prolonged use should be avoided.
As with other antacid products, taking this product can mask the symptoms of other more serious, underlying medical conditions.
Contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) which may cause allergic reactions (possibly delayed).
Tablet: The sodium content of a two-tablet dose is 110.75 mg (4.82 mmol). This should be taken into account when a highly restricted salt diet is recommended, e.g. in some cases of congestive cardiac failure and renal impairment.
Each two-tablet dose contains 150 mg (3.75 mmol) of calcium. Care needs to be taken in treating patients with hypercalcaemia, nephrocalcinosis and recurrent calcium containing renal calculi.
Treatment of children younger than 12 years of age is not generally recommended, except on medical advice.
If symptoms do not improve after seven days, the clinical situation should be reviewed. Prolonged use should be avoided.
As with other antacid products, taking this product can mask the symptoms of other more serious, underlying medical conditions.
Due to its aspartame content this product should not be given to patients with phenylketonuria.
Effects on Ability to Drive and Use Machines: This product has no or negligible influence on the ability to drive and use machines.
Each 10 mL dose contains 130 mg (3.25 mmol) of calcium. Care needs to be taken in treating patients with hypercalcaemia, nephrocalcinosis and recurrent calcium containing renal calculi.
Treatment of children younger than 12 years of age is not generally recommended, except on medical advice.
If symptoms do not improve after seven days, the clinical situation should be reviewed. Prolonged use should be avoided.
As with other antacid products, taking this product can mask the symptoms of other more serious, underlying medical conditions.
Contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) which may cause allergic reactions (possibly delayed).
Tablet: The sodium content of a two-tablet dose is 110.75 mg (4.82 mmol). This should be taken into account when a highly restricted salt diet is recommended, e.g. in some cases of congestive cardiac failure and renal impairment.
Each two-tablet dose contains 150 mg (3.75 mmol) of calcium. Care needs to be taken in treating patients with hypercalcaemia, nephrocalcinosis and recurrent calcium containing renal calculi.
Treatment of children younger than 12 years of age is not generally recommended, except on medical advice.
If symptoms do not improve after seven days, the clinical situation should be reviewed. Prolonged use should be avoided.
As with other antacid products, taking this product can mask the symptoms of other more serious, underlying medical conditions.
Due to its aspartame content this product should not be given to patients with phenylketonuria.
Effects on Ability to Drive and Use Machines: This product has no or negligible influence on the ability to drive and use machines.
Use In Pregnancy & Lactation
Use in Pregnancy: Clinical studies in more than 500 pregnant women as well as a large amount of data from post-marketing experience indicate no malformative or feto/neonatal toxicity of the active substances.
This product can be used during pregnancy, if clinically needed.
Taking into account the presence of calcium carbonate, it is recommended to limit the treatment duration as much as possible.
Use in Lactation: No effects of the active substances have been shown in breastfed newborns/infants of treated mothers. This product can be used during breast-feeding.
Fertility: Clinical data do not suggest that this product has an effect on human fertility.
This product can be used during pregnancy, if clinically needed.
Taking into account the presence of calcium carbonate, it is recommended to limit the treatment duration as much as possible.
Use in Lactation: No effects of the active substances have been shown in breastfed newborns/infants of treated mothers. This product can be used during breast-feeding.
Fertility: Clinical data do not suggest that this product has an effect on human fertility.
Adverse Reactions
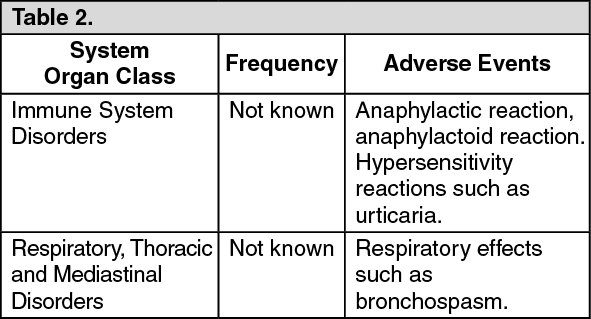
Hypersensitivity reactions (including anaphylaxis and urticaria) and respiratory effects (including bronchospasm) have been reported very rarely.
Adverse events which have been associated with sodium alginate, sodium bicarbonate and calcium carbonate are given as follows, tabulated by system organ class and frequency. Frequencies are defined as: Very common (≥1/10); common (≥1/100 and <1/10); uncommon (≥1/1000 and <1/100); rare (≥1/10,000 and <1/1000); very rare (<1/10,000); not known (cannot be estimated from the available data). Within each frequency grouping, adverse events are presented in order of decreasing seriousness. (See Table 2.)
Adverse events which have been associated with sodium alginate, sodium bicarbonate and calcium carbonate are given as follows, tabulated by system organ class and frequency. Frequencies are defined as: Very common (≥1/10); common (≥1/100 and <1/10); uncommon (≥1/1000 and <1/100); rare (≥1/10,000 and <1/1000); very rare (<1/10,000); not known (cannot be estimated from the available data). Within each frequency grouping, adverse events are presented in order of decreasing seriousness. (See Table 2.)
Storage
Store below 30°C.
Action
Pharmacotherapeutic Group: Other drugs for peptic ulcer and gastro-oesophageal reflux disease (GORD). ATC Code: A02BX.
Pharmacology: Pharmacodynamics: The medicinal product is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate.
On ingestion, the medicinal product reacts rapidly with gastric acid to form a raft alginic acid gel having a near neutral pH and which floats on the stomach contents effectively impeding gastro-oesophageal reflux. In severe cases, the raft itself may be refluxed into the oesophagus, in preference to the stomach contents, and exert a demulcent effect.
Calcium carbonate neutralises gastric acid to provide fast relief from indigestion and heartburn. This effect is increased by the addition of sodium bicarbonate which also has a neutralising action. The total neutralising capacity of the product at the lowest dose of two tablets or 10 mL dose is approximately 10 mEqH+.
Pharmacokinetics: The mode of action of the medicinal product is physical and does not depend on absorption into the systemic circulation.
Toxicology: Preclinical Safety Data: There are no preclinical safety data of relevance to the consumer.
Pharmacology: Pharmacodynamics: The medicinal product is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate.
On ingestion, the medicinal product reacts rapidly with gastric acid to form a raft alginic acid gel having a near neutral pH and which floats on the stomach contents effectively impeding gastro-oesophageal reflux. In severe cases, the raft itself may be refluxed into the oesophagus, in preference to the stomach contents, and exert a demulcent effect.
Calcium carbonate neutralises gastric acid to provide fast relief from indigestion and heartburn. This effect is increased by the addition of sodium bicarbonate which also has a neutralising action. The total neutralising capacity of the product at the lowest dose of two tablets or 10 mL dose is approximately 10 mEqH+.
Pharmacokinetics: The mode of action of the medicinal product is physical and does not depend on absorption into the systemic circulation.
Toxicology: Preclinical Safety Data: There are no preclinical safety data of relevance to the consumer.
MedsGo Class
Antacids, Antireflux Agents & Antiulcerants
Features
Dosage
250 mg / 106.5 mg / 187.5 mg
Ingredients
- Alginate
- Bicarbonate
- Calcium
Packaging
Chewable Tablet 8's
Generic Name
Alginate / Bicarbonate / Calcium
Drug Flavor
Mixed Berries
Registration Number
DR-XY46902
Classification
Over-The-Counter (OTC)
Reviews
No reviews found
Product Questions
Questions


