All variations
Indications / Uses
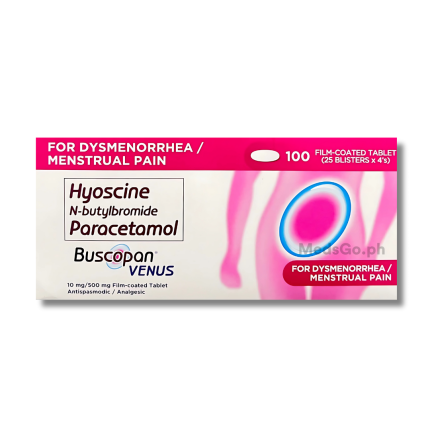
BUSCOPAN VENUS is used for the treatment of abdominal pain and discomfort associated with cramps of the gastrointestinal tract and biliary system. It is specifically effective for managing paroxysmal pain in diseases of the stomach or intestine, as well as spastic conditions of the female genital tract, such as dysmenorrhea. By combining an antispasmodic with an analgesic, it provides dual-action relief from both the spasm itself and the resulting pain.
Formulation / Ingredients
BUSCOPAN VENUS contains: Hyoscine-N-Butylbromide 10mg + Paracetamol 500mg
Do I need prescription to buy BUSCOPAN VENUS?
No prescription required to purchase BUSCOPAN VENUS. You can order it right now on our website.
Side Effects
BUSCOPAN VENUS common side effects include: dry mouth, skin reactions such as sweating or itching, and tachycardia. Some users may experience dizziness, fatigue, or visual disturbances. In rare cases, serious allergic reactions like thrombocytopenia, leukopenia, or anaphylactic shock have been reported. Minor gastrointestinal issues and urinary retention may also occur.
Directions and Dosage
- Adults (18+ years): 1 to 2 tablets, 3 times daily. Do not exceed 6 tablets in 24 hours.
- Adolescents 12–18 years: 1 tablet, 3 times daily. Do not exceed 3 tablets in 24 hours.
Not recommended for children under 10 years. Maximum treatment duration: 3 days for fever or 5 days for pain unless otherwise directed. Tablets should be swallowed whole with enough water.
Contraindications
Avoid use in cases of mechanical stenosis of the gastrointestinal tract, paralytic ileus, or intestinal atony. It is strictly prohibited for patients with myasthenia gravis, megacolon, glaucoma, or severe hepatic and renal insufficiency.
Special Precautions
- Use with caution if you have underlying liver or kidney dysfunction.
- Extra care is needed for individuals with glucose-6-phosphate dehydrogenase deficiency.
- Avoid chronic use or high doses as it may lead to liver damage.
Is it safe to take it with other medications?
Concurrent use with alcohol, anticoagulants, or other products containing paracetamol should be avoided. Interactions may occur with metoclopramide or antidepressants, potentially altering the effect of the medication.
How should I store it?
Store in a dry place at temperatures not exceeding 30°C. Keep the tablets in their original packaging to protect from moisture and light.
Used For
- Menstrual cramps, Spasm
Age
- 10 years & up
Features
- Hyoscine N-Butylbromide
- Paracetamol
Reviews
No reviews found
Pharmacist answers to questions about BUSCOPAN VENUS 1 Tablet - Hyoscine-N-Butylbromide / Paracetamol 10mg / 500mg