All variations
Indications / Uses
SOLMUX BRONCHO is used for treating respiratory conditions with bronchospasm and excessive mucus production such as acute bronchitis, chronic bronchitis, and COPD. It combines bronchodilator action with mucolytic effects to ease breathing difficulties and improve mucus clearance. This dual-action formula helps manage productive coughing and airway obstruction in inflammatory respiratory disorders.
Formulation / Ingredients
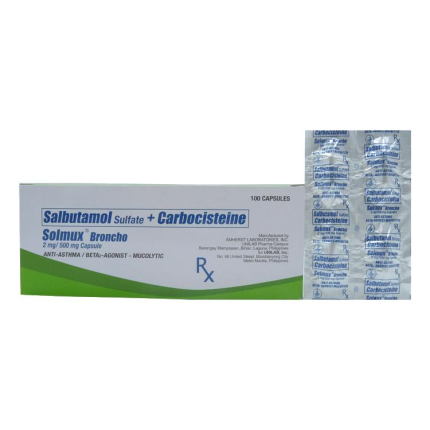
SOLMUX BRONCHO contains: Salbutamol 2mg + Carbocisteine 500mg.
Do I need prescription to buy SOLMUX BRONCHO?
Yes, you need a prescription. But if you don't have one, you can get a free teleconsultation from MedsGo Physician. Just make an order and our Physician will call you back.
Side Effects
SOLMUX BRONCHO common side effects include: tremors, nausea, headache, and rapid heartbeat. Less frequently, gastrointestinal discomfort, muscle cramps, or throat irritation may occur. These reactions are typically mild and transient with continued use.
Directions and Dosage
- Adults and adolescents (12+ years): 1 capsule 3 times daily. Maximum: 3 capsules daily.
- Children 6-11 years: ½ capsule 3 times daily. Maximum: 1.5 capsules daily.
Not recommended for children under 6. Treatment duration typically ranges from 5-7 days based on symptom improvement.
Contraindications
Hypersensitivity to components. Severe cardiac disorders like tachyarrhythmias. Active peptic ulcer disease or gastrointestinal bleeding.
Use in Pregnancy and Lactation
Risk-benefit assessment required. Components may cross placental barrier or excrete in breast milk.
Special Precautions
- Cardiovascular disorders require monitoring
- Diabetes management may need adjustment
- Hepatic impairment necessitates dosage review
Is it safe to take it with other medications?
Beta-blockers may reduce bronchodilator effectiveness. Diuretics can increase hypokalemia risk. Avoid concurrent use with cough suppressants.
How should I store it?
Store below 30°C in original packaging. Keep away from moisture and light. Ensure container is tightly sealed.
Used For
Bronchitis, COPD
Age
6+ years
Features
- Carbocisteine
- Salbutamol
Reviews
No reviews found
Pharmacist answers to questions about SOLMUX BRONCHO Salbutamol Sulfate / Carbocisteine 2mg / 500mg Capsule 1's