Indications/Uses
Testosterone replacement in primary and secondary male hypogonadism.
Dosage/Direction for Use
Method of administration: Solution for injection.
Dosage regimen: Testosterone undecanoate (Nebido) (1 vial corresponding to 1000 mg testosterone undecanoate) is injected every 10 to 14 weeks. Injections with this frequency are capable of maintaining sufficient testosterone levels and do not lead to accumulation.
The injections must be administered very slowly. Testosterone undecanoate (Nebido) is strictly for intramuscular injection. Special care must be given to avoid intravasal injection. See Instructions for use/handling under Cautions for Usage to avoid injury when opening.
Start of treatment: Serum testosterone levels should be measured before start of treatment. The first injection interval may be reduced to a minimum of 6 weeks. With this loading dose, steady-state levels will be reached quickly.
Individualization of treatment: It is advisable to measure testosterone serum levels occasionally at the end of an injection interval. Serum levels below normal range would indicate the need for a shorter injection interval. In case of high serum levels an extension of the injection interval may be considered. The injection interval should remain within the recommended range of 10 to 14 weeks.
Additional information on special populations: Pediatric patients: Testosterone undecanoate (Nebido) is not indicated for use in children and adolescents and it has not been clinically evaluated in males under 18 years of age (see Precautions).
Geriatric patients: Limited data do not suggest the need for a dosage adjustment in elderly patients (see Precautions).
Patients with hepatic impairment: No formal studies have been performed in patients with hepatic impairment. The use of Testosterone undecanoate (Nebido) is contraindicated in men with past or present liver tumors (see Contraindications).
Patients with renal impairment: No formal studies have been performed in patients with renal impairment.
Dosage regimen: Testosterone undecanoate (Nebido) (1 vial corresponding to 1000 mg testosterone undecanoate) is injected every 10 to 14 weeks. Injections with this frequency are capable of maintaining sufficient testosterone levels and do not lead to accumulation.
The injections must be administered very slowly. Testosterone undecanoate (Nebido) is strictly for intramuscular injection. Special care must be given to avoid intravasal injection. See Instructions for use/handling under Cautions for Usage to avoid injury when opening.
Start of treatment: Serum testosterone levels should be measured before start of treatment. The first injection interval may be reduced to a minimum of 6 weeks. With this loading dose, steady-state levels will be reached quickly.
Individualization of treatment: It is advisable to measure testosterone serum levels occasionally at the end of an injection interval. Serum levels below normal range would indicate the need for a shorter injection interval. In case of high serum levels an extension of the injection interval may be considered. The injection interval should remain within the recommended range of 10 to 14 weeks.
Additional information on special populations: Pediatric patients: Testosterone undecanoate (Nebido) is not indicated for use in children and adolescents and it has not been clinically evaluated in males under 18 years of age (see Precautions).
Geriatric patients: Limited data do not suggest the need for a dosage adjustment in elderly patients (see Precautions).
Patients with hepatic impairment: No formal studies have been performed in patients with hepatic impairment. The use of Testosterone undecanoate (Nebido) is contraindicated in men with past or present liver tumors (see Contraindications).
Patients with renal impairment: No formal studies have been performed in patients with renal impairment.
Overdosage
No special therapeutic measure apart from termination of therapy with the drug or dose reduction is necessary after overdosage.
Contraindications
Androgen-dependent carcinoma of the prostate or of the male mammary gland.
Hypercalcemia accompanying malignant tumors.
Past or present liver tumors.
Hypersensitivity to the active substance or to any of the excipients.
The use of Testosterone undecanoate (Nebido) in women is contraindicated.
Hypercalcemia accompanying malignant tumors.
Past or present liver tumors.
Hypersensitivity to the active substance or to any of the excipients.
The use of Testosterone undecanoate (Nebido) in women is contraindicated.
Special Precautions
Older patients treated with androgens may be at an increased risk for the development of prostatic hyperplasia. Although there are no clear indications that androgens actually generate prostatic carcinoma, these can enhance the growth of any existing prostatic carcinoma. Therefore carcinoma of the prostate has to be excluded before starting therapy with testosterone preparations.
As a precaution, regular examinations of the prostate are recommended in men.
Hemoglobin and hematocrit should be checked periodically in patients on long-term androgen therapy to detect cases of polycythemia (see Adverse Reactions).
As a general rule, the risk of bleeding from using intramuscular injections in patients with acquired or inherited bleeding disorders always has to be taken into account. Testosterone and derivatives have been reported to increase the activity of coumarin derived oral anticoagulants (see also Interactions).
Testosterone should be used with caution in patients with thrombophilia, as there have been post-marketing studies and reports of thrombotic events in these patients during testosterone therapy.
Cases of benign and malignant liver tumors have been reported in users of hormonal substances, such as androgen compounds. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal hemorrhage occur in men using Testosterone undecanoate (Nebido), a liver tumor should be included in the differential-diagnostic considerations.
Caution should be exercised in patients predisposed to edema, e.g. in case of severe cardiac, hepatic, or renal insufficiency or ischemic heart disease, as treatment with androgens may result in increased retention of sodium and water. In case of severe complications characterized by edema with or without congestive heart failure, treatment must be stopped immediately (see Adverse Reactions).
Testosterone may cause a rise in blood pressure and Testosterone undecanoate (Nebido) should be used with caution in men with hypertension.
Clinical trials with Testosterone undecanoate (Nebido) in children or adolescents under the age of 18 have so far not been conducted.
In children testosterone, besides masculinization, can cause accelerated growth, bone maturation and premature epiphyseal closure, thereby reducing final height. The appearance of common acne has to be expected.
Preexisting sleep apnea may be potentiated.
Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication(s) and in combination with other anabolic androgenic steroids.
Testosterone abuse may result in dependence and withdrawal symptoms upon significant dose reduction or abrupt discontinuation of use.
Abuse of testosterone along with other anabolic androgenic steroids can lead to serious adverse reactions including: cardiovascular (with fatal outcomes in some cases), hepatic and/or psychiatric events.
As with all oily solutions, Testosterone undecanoate (Nebido) must be injected strictly intramuscularly and very slowly. Pulmonary microembolism of oily solutions can in rare cases lead to signs and symptoms such as cough, dyspnoea, malaise, hyperhydrosis, chest pain, dizziness, paraesthesia, or syncope. These reactions may occur during or immediately after the injection and are reversible. Treatment is usually supportive, e.g. by administration of supplemental oxygen.
Suspected anaphylactic reactions after Testosterone undecanoate (Nebido) injection have been reported.
Effects on ability to drive or use machines: No observed effects.
As a precaution, regular examinations of the prostate are recommended in men.
Hemoglobin and hematocrit should be checked periodically in patients on long-term androgen therapy to detect cases of polycythemia (see Adverse Reactions).
As a general rule, the risk of bleeding from using intramuscular injections in patients with acquired or inherited bleeding disorders always has to be taken into account. Testosterone and derivatives have been reported to increase the activity of coumarin derived oral anticoagulants (see also Interactions).
Testosterone should be used with caution in patients with thrombophilia, as there have been post-marketing studies and reports of thrombotic events in these patients during testosterone therapy.
Cases of benign and malignant liver tumors have been reported in users of hormonal substances, such as androgen compounds. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal hemorrhage occur in men using Testosterone undecanoate (Nebido), a liver tumor should be included in the differential-diagnostic considerations.
Caution should be exercised in patients predisposed to edema, e.g. in case of severe cardiac, hepatic, or renal insufficiency or ischemic heart disease, as treatment with androgens may result in increased retention of sodium and water. In case of severe complications characterized by edema with or without congestive heart failure, treatment must be stopped immediately (see Adverse Reactions).
Testosterone may cause a rise in blood pressure and Testosterone undecanoate (Nebido) should be used with caution in men with hypertension.
Clinical trials with Testosterone undecanoate (Nebido) in children or adolescents under the age of 18 have so far not been conducted.
In children testosterone, besides masculinization, can cause accelerated growth, bone maturation and premature epiphyseal closure, thereby reducing final height. The appearance of common acne has to be expected.
Preexisting sleep apnea may be potentiated.
Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication(s) and in combination with other anabolic androgenic steroids.
Testosterone abuse may result in dependence and withdrawal symptoms upon significant dose reduction or abrupt discontinuation of use.
Abuse of testosterone along with other anabolic androgenic steroids can lead to serious adverse reactions including: cardiovascular (with fatal outcomes in some cases), hepatic and/or psychiatric events.
As with all oily solutions, Testosterone undecanoate (Nebido) must be injected strictly intramuscularly and very slowly. Pulmonary microembolism of oily solutions can in rare cases lead to signs and symptoms such as cough, dyspnoea, malaise, hyperhydrosis, chest pain, dizziness, paraesthesia, or syncope. These reactions may occur during or immediately after the injection and are reversible. Treatment is usually supportive, e.g. by administration of supplemental oxygen.
Suspected anaphylactic reactions after Testosterone undecanoate (Nebido) injection have been reported.
Effects on ability to drive or use machines: No observed effects.
Use In Pregnancy & Lactation
Pregnancy: Not applicable.
Lactation: Not applicable.
Fertility: Testosterone replacement therapy may reversibly reduce spermatogenesis (see Adverse Reactions and Pharmacology: Toxicology: Preclinical safety data).
Lactation: Not applicable.
Fertility: Testosterone replacement therapy may reversibly reduce spermatogenesis (see Adverse Reactions and Pharmacology: Toxicology: Preclinical safety data).
Adverse Reactions
Summary of the safety profile: Regarding undesirable effects associated with the use of androgens, please also refer to Precautions.
The most frequently reported undesirable effects during treatment with Testosterone undecanoate (Nebido) are acne and injection site pain.
The table as follows reports adverse drug reactions (ADRs) by MedDRA system organ classes (MedDRA SOCs, version 10.1)* reported with Testosterone undecanoate (Nebido). The frequencies are based on clinical trial data and defined as Common (≥1/100 to <1/10) and Uncommon (≥1/1000 to <1/100). The ADRs were recorded in 6 clinical studies (N=422) and considered at least possibly causally related to Testosterone undecanoate (Nebido).
Tabulated list of adverse reactions: See table.
The most frequently reported undesirable effects during treatment with Testosterone undecanoate (Nebido) are acne and injection site pain.
The table as follows reports adverse drug reactions (ADRs) by MedDRA system organ classes (MedDRA SOCs, version 10.1)* reported with Testosterone undecanoate (Nebido). The frequencies are based on clinical trial data and defined as Common (≥1/100 to <1/10) and Uncommon (≥1/1000 to <1/100). The ADRs were recorded in 6 clinical studies (N=422) and considered at least possibly causally related to Testosterone undecanoate (Nebido).
Tabulated list of adverse reactions: See table.
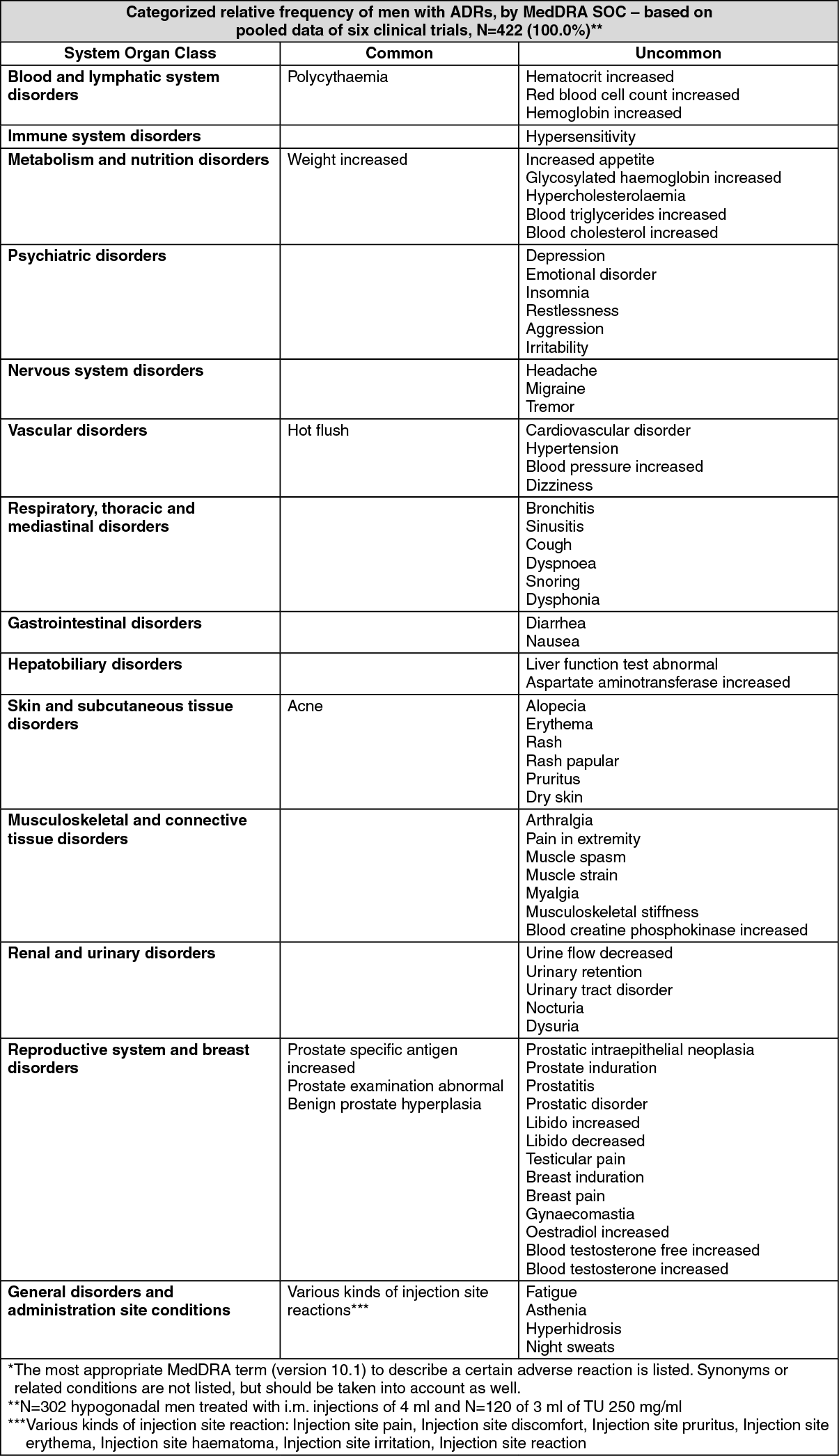
Description of selected adverse reactions: Pulmonary microembolism of oily solutions can in rare cases lead to signs and symptoms such as cough, dyspnoea, malaise, hyperhydrosis, chest pain, dizziness, paresthesia, or syncope. These reactions may occur during or immediately after the injections and are reversible. Cases suspected by the company or the reporter to represent oily pulmonary microembolism have been reported rarely in clinical trials (in ≥1/10,000 and <1/1,000 injections) as well as from postmarketing experience (see Precautions).
Suspected anaphylactic reactions after Testosterone undecanoate (Nebido) injection have been reported.
In addition to the previously mentioned adverse reactions, nervousness, hostility, sleep apnea, various skin reactions including seborrhoea, increased hair growth, increased frequency of erections and in very rare cases jaundice have been reported under treatment with testosterone containing preparations.
Therapy with high doses of testosterone preparations commonly reversibly interrupts or reduces spermatogenesis, thereby reducing the size of the testicles; testosterone replacement therapy of hypogonadism can in rare cases cause persistent, painful erections (priapism). High-dosed or long-term administration of testosterone occasionally increases the occurrences of water retention and edema.
Suspected anaphylactic reactions after Testosterone undecanoate (Nebido) injection have been reported.
In addition to the previously mentioned adverse reactions, nervousness, hostility, sleep apnea, various skin reactions including seborrhoea, increased hair growth, increased frequency of erections and in very rare cases jaundice have been reported under treatment with testosterone containing preparations.
Therapy with high doses of testosterone preparations commonly reversibly interrupts or reduces spermatogenesis, thereby reducing the size of the testicles; testosterone replacement therapy of hypogonadism can in rare cases cause persistent, painful erections (priapism). High-dosed or long-term administration of testosterone occasionally increases the occurrences of water retention and edema.
Drug Interactions
Drugs that affect testosterone: Barbiturates and other enzyme inducers: Interactions can occur with drugs that induce microsomal enzymes which can result in increased clearance of testosterone.
Effects of androgens on other drugs: Oxyphenbutazone: Increased oxyphenbutazone serum levels have been reported.
Oral anticoagulants: Testosterone and its derivatives have been reported to increase the activity of coumarin derived oral anticoagulants, possibly requiring dose adjustment. Independent of this finding, the risk of bleeding from using intramuscular injections in patients with acquired or inherited bleeding disorders always has to be taken into account as a general rule.
Hypoglycaemics: Androgens may enhance the blood sugar reducing effects of insulin. Therefore, the dosage of hypoglycaemic agents may need to be lowered.
Effects of androgens on other drugs: Oxyphenbutazone: Increased oxyphenbutazone serum levels have been reported.
Oral anticoagulants: Testosterone and its derivatives have been reported to increase the activity of coumarin derived oral anticoagulants, possibly requiring dose adjustment. Independent of this finding, the risk of bleeding from using intramuscular injections in patients with acquired or inherited bleeding disorders always has to be taken into account as a general rule.
Hypoglycaemics: Androgens may enhance the blood sugar reducing effects of insulin. Therefore, the dosage of hypoglycaemic agents may need to be lowered.
Caution For Usage
Incompatibilities: In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
Instructions for use/handling: Vial: The vial is for single use only. The content of a vial is to be injected intramuscularly immediately after drawing up into the syringe. After removal of the plastic cap do not remove the metal ring or the crimp cap.
Instructions for use/handling: Vial: The vial is for single use only. The content of a vial is to be injected intramuscularly immediately after drawing up into the syringe. After removal of the plastic cap do not remove the metal ring or the crimp cap.
Storage
Store at temperatures not exceeding 30°C.
Action
Pharmacotherapeutic group: Androgens, 3-oxoandrosten (4) derivatives. ATC Code: G03BA03.
Pharmacology: Pharmacodynamics: Testosterone undecanoate is an ester of the naturally occurring androgen, testosterone. The active form, testosterone, is formed by cleavage of the side chain.
Testosterone is the most important androgen of the male, mainly synthesized in the testicles, and to a small extent in the adrenal cortex.
Testosterone is responsible for the expression of masculine characteristics during fetal, early childhood, and pubertal development and thereafter for maintaining the masculine phenotype and androgen-dependent functions (e.g. spermatogenesis, accessory sexual glands).
Insufficient secretion of testosterone results in male hypogonadism characterized by low serum testosterone concentrations. Signs and symptoms associated with male hypogonadism include but are not limited to, erectile dysfunction and decreased sexual desire, fatigue, depressive moods as well as a lacking of secondary sexual characteristics, their incomplete development, or their regression, an increased risk of osteoporosis, an increase of visceral fat and a decrease of lean body mass and muscle strength. Exogenous androgens are given to improve the deficient endogenous testosterone levels and related signs and symptoms.
Dependent on the target organ, the spectrum of activities of testosterone is mainly androgenic (e.g. prostate, seminal vesicles, epididymis) or protein-anabolic (muscle, bone, hematopoiesis, kidney, liver).
The effects of testosterone in some organs arise after peripheral conversion of testosterone to estradiol, which than binds to estrogen receptors in the target cell nucleus e.g. the pituitary, fat, brain, bone, and testicular Leydig cells.
In hypogonadal men androgens decrease the body fat mass, increase the body lean mass, muscle strength, and prevent bone loss. Androgens may improve sexual function and also may exert positive psychotropic effects by improving mood.
Pharmacokinetics: Absorption: Testosterone undecanoate (Nebido) is an intramuscularly administered depot preparation of testosterone undecanoate and thus circumvents the first-pass effect. Following intramuscular injection of testosterone undecanoate as an oily solution, the compound is gradually released from the depot and is almost completely cleaved by serum esterases into testosterone and undecanoic acid.
Distribution: In two separate studies, mean maximum concentrations of testosterone of 24 and 45 nmol/L were measured about 14 and 7 days, respectively, after single i.m. administration of 1000 mg of testosterone undecanoate to hypogonadal men. Post-maximum testosterone levels declined with an estimated half-life of about 53 days.
Following intravenous infusion of testosterone to elderly men, an apparent volume of distribution of about 1.0 L/kg was determined.
Metabolism/Biotransformation: Testosterone which is generated by ester cleavage from testosterone undecanoate is metabolized and excreted the same way as endogenous testosterone. The undecanoic acid is metabolized by β-oxidation in the same way as other aliphatic carboxylic acids.
Elimination/Excretion: Testosterone undergoes extensive hepatic and extrahepatic metabolism. After the administration of radiolabeled testosterone, about 90% of the radioactivity appears in the urine as glucuronic and sulphuric acid conjugates and 6% appears in the feces after undergoing enterohepatic circulation. Urinary products include androsterone and etiocholanolone.
Steady state conditions: Following repeated i.m. injection of 1000 mg testosterone undecanoate to hypogonadal men using an interval of 10 weeks between two injections, steady state conditions were achieved between the 3rd and the 5th administration. Mean Cmax and Cmin values of testosterone at steady state were about 42 and 17 nmol/L, respectively. Post-maximum testosterone levels in the serum decreased with a half-life of about 90 days, which corresponds to the release rate from the depot.
Toxicology: Preclinical safety data: Systemic toxicity: Acute toxicity: As with steroid hormones in general, the acute toxicity of testosterone is very low.
Chronic toxicity: No effects which might indicate an unexpected risk to humans were observed during systemic toxicity studies in a rodent or non-rodent species after repeated administration of either the undecanoate or the enanthate ester of testosterone.
Mutagenic and tumorigenic potential: In vitro and in vivo investigations into a mutagenic effect of testosterone undecanoate itself as well as studies on testosterone itself gave no indications of a mutagenic potential.
Studies in rodents indicate a promoting effect of testosterone or of its esters on the development of hormone-dependent tumors. Generally, it should be born in mind that sex steroids can promote the growth of certain hormone-dependent tissues and tumors.
Reproduction toxicity: Fertility studies in rodents and primates have shown that treatment with testosterone can impair fertility by suppressing spermatogenesis in a dose-dependent manner. Furthermore, no embryolethal or teratogenic effects were observed in the offspring of testosterone-treated male rats. Administration of Testosterone undecanoate (Nebido) may cause virilization of female fetuses in certain development stages. However, investigations into the embryotoxic, in particular teratogenic, effects gave no indication that further impairment of organ development is to be expected.
Local tolerance and contact sensitizing potential: The local tolerance study on pigs following intramuscular administration showed that Testosterone undecanoate (Nebido) does not increase the irritative effects already caused by the solvent. The solvent of Testosterone undecanoate (Nebido) has been used for many years in numerous formulations for human use. In this time no local irritative effects have been observed which would object to its further use.
Pharmacology: Pharmacodynamics: Testosterone undecanoate is an ester of the naturally occurring androgen, testosterone. The active form, testosterone, is formed by cleavage of the side chain.
Testosterone is the most important androgen of the male, mainly synthesized in the testicles, and to a small extent in the adrenal cortex.
Testosterone is responsible for the expression of masculine characteristics during fetal, early childhood, and pubertal development and thereafter for maintaining the masculine phenotype and androgen-dependent functions (e.g. spermatogenesis, accessory sexual glands).
Insufficient secretion of testosterone results in male hypogonadism characterized by low serum testosterone concentrations. Signs and symptoms associated with male hypogonadism include but are not limited to, erectile dysfunction and decreased sexual desire, fatigue, depressive moods as well as a lacking of secondary sexual characteristics, their incomplete development, or their regression, an increased risk of osteoporosis, an increase of visceral fat and a decrease of lean body mass and muscle strength. Exogenous androgens are given to improve the deficient endogenous testosterone levels and related signs and symptoms.
Dependent on the target organ, the spectrum of activities of testosterone is mainly androgenic (e.g. prostate, seminal vesicles, epididymis) or protein-anabolic (muscle, bone, hematopoiesis, kidney, liver).
The effects of testosterone in some organs arise after peripheral conversion of testosterone to estradiol, which than binds to estrogen receptors in the target cell nucleus e.g. the pituitary, fat, brain, bone, and testicular Leydig cells.
In hypogonadal men androgens decrease the body fat mass, increase the body lean mass, muscle strength, and prevent bone loss. Androgens may improve sexual function and also may exert positive psychotropic effects by improving mood.
Pharmacokinetics: Absorption: Testosterone undecanoate (Nebido) is an intramuscularly administered depot preparation of testosterone undecanoate and thus circumvents the first-pass effect. Following intramuscular injection of testosterone undecanoate as an oily solution, the compound is gradually released from the depot and is almost completely cleaved by serum esterases into testosterone and undecanoic acid.
Distribution: In two separate studies, mean maximum concentrations of testosterone of 24 and 45 nmol/L were measured about 14 and 7 days, respectively, after single i.m. administration of 1000 mg of testosterone undecanoate to hypogonadal men. Post-maximum testosterone levels declined with an estimated half-life of about 53 days.
Following intravenous infusion of testosterone to elderly men, an apparent volume of distribution of about 1.0 L/kg was determined.
Metabolism/Biotransformation: Testosterone which is generated by ester cleavage from testosterone undecanoate is metabolized and excreted the same way as endogenous testosterone. The undecanoic acid is metabolized by β-oxidation in the same way as other aliphatic carboxylic acids.
Elimination/Excretion: Testosterone undergoes extensive hepatic and extrahepatic metabolism. After the administration of radiolabeled testosterone, about 90% of the radioactivity appears in the urine as glucuronic and sulphuric acid conjugates and 6% appears in the feces after undergoing enterohepatic circulation. Urinary products include androsterone and etiocholanolone.
Steady state conditions: Following repeated i.m. injection of 1000 mg testosterone undecanoate to hypogonadal men using an interval of 10 weeks between two injections, steady state conditions were achieved between the 3rd and the 5th administration. Mean Cmax and Cmin values of testosterone at steady state were about 42 and 17 nmol/L, respectively. Post-maximum testosterone levels in the serum decreased with a half-life of about 90 days, which corresponds to the release rate from the depot.
Toxicology: Preclinical safety data: Systemic toxicity: Acute toxicity: As with steroid hormones in general, the acute toxicity of testosterone is very low.
Chronic toxicity: No effects which might indicate an unexpected risk to humans were observed during systemic toxicity studies in a rodent or non-rodent species after repeated administration of either the undecanoate or the enanthate ester of testosterone.
Mutagenic and tumorigenic potential: In vitro and in vivo investigations into a mutagenic effect of testosterone undecanoate itself as well as studies on testosterone itself gave no indications of a mutagenic potential.
Studies in rodents indicate a promoting effect of testosterone or of its esters on the development of hormone-dependent tumors. Generally, it should be born in mind that sex steroids can promote the growth of certain hormone-dependent tissues and tumors.
Reproduction toxicity: Fertility studies in rodents and primates have shown that treatment with testosterone can impair fertility by suppressing spermatogenesis in a dose-dependent manner. Furthermore, no embryolethal or teratogenic effects were observed in the offspring of testosterone-treated male rats. Administration of Testosterone undecanoate (Nebido) may cause virilization of female fetuses in certain development stages. However, investigations into the embryotoxic, in particular teratogenic, effects gave no indication that further impairment of organ development is to be expected.
Local tolerance and contact sensitizing potential: The local tolerance study on pigs following intramuscular administration showed that Testosterone undecanoate (Nebido) does not increase the irritative effects already caused by the solvent. The solvent of Testosterone undecanoate (Nebido) has been used for many years in numerous formulations for human use. In this time no local irritative effects have been observed which would object to its further use.
MedsGo Class
Androgens & Related Synthetic Drugs
Features
Dosage
250 mg / ml (1 g / 4 ml)
Ingredients
- Testosterone undecanoate
Packaging
Solution for Injection (I.M.) 4ml x 1's
Generic Name
Testosterone undecanoate
Registration Number
DR-XY45872
Classification
Prescription Drug (RX)
Reviews
No reviews found
Pharmacist answers to questions about NEBIDO Testosterone Undecanoate 250mg / mL Solution for IM Injection 4mL 1's
Questions


