All variations
Indications/Uses
Progesterone deficiencies: Treatment of dysmenorrhea.
Treatment of endometriosis.
Treatment of secondary amenorrhea.
Treatment of irregular cycles.
Treatment of dysfunctional uterine bleeding.
Treatment of pre-menstrual syndrome.
Treatment of threatened miscarriage.
Treatment of habitual miscarriage.
Treatment of infertility due to luteal insufficiency.
Luteal support as part of an Assisted Reproductive Technology (ART) treatment.
Hormone replacement therapy: To counteract the effects of unopposed estrogen on the endometrium in hormone replacement therapy for women with disorders due to natural or surgical induced menopause with an intact uterus.
Treatment of endometriosis.
Treatment of secondary amenorrhea.
Treatment of irregular cycles.
Treatment of dysfunctional uterine bleeding.
Treatment of pre-menstrual syndrome.
Treatment of threatened miscarriage.
Treatment of habitual miscarriage.
Treatment of infertility due to luteal insufficiency.
Luteal support as part of an Assisted Reproductive Technology (ART) treatment.
Hormone replacement therapy: To counteract the effects of unopposed estrogen on the endometrium in hormone replacement therapy for women with disorders due to natural or surgical induced menopause with an intact uterus.
Dosage/Direction for Use
Dosages, treatment schedule and duration of treatment may be adapted to the severity of the dysfunction and the clinical response.
Dysmenorrhea: 10 or 20 mg dydrogesterone per day from day 5 to day 25 of the menstrual cycle.
Endometriosis: 10 to 30 mg dydrogesterone per day from day 5 to day 25 of the cycle or continuously.
Dysfunctional uterine bleeding: When treatment is started to arrest a bleeding episode, 20 or 30 mg dydrogesterone per day is to be given for up to 10 days.
For continuous treatment, 10 or 20 mg dydrogesterone per day should be given during the second half of the menstrual cycle. The starting day and the number of treatment days will depend on the individual cycle length.
Withdrawal bleeding occurs if the endometrium has been adequately primed with either endogenous or exogenous estrogen.
Secondary amenorrhea: 10 or 20 mg dydrogesterone per day, to be given daily for 14 days during the second half of the theoretical menstrual cycle to produce an optimum secretory transformation of an endometrium that has been adequately primed with either endogenous or exogenous estrogen.
Pre-menstrual syndrome: 10 mg dydrogesterone twice daily starting with the second half of the menstrual cycle until the first day of the next cycle. The starting day and the number of treatment days will depend on the individual cycle length.
Irregular cycles: 10 or 20 mg dydrogesterone per day starting with the second half of the menstrual cycle until the first day of the next cycle. The starting day and the number of treatment days will depend on the individual cycle length.
Threatened miscarriage: An initial dose of up to 40 mg dydrogesterone may be given followed by 20 or 30mg per day until symptoms remit.
Habitual miscarriage: 10 mg dydrogesterone twice daily until the twelfth week of pregnancy.
Infertility due to luteal insufficiency: 10 or 20 mg dydrogesterone daily starting with the second half of the menstrual cycle until the first day of the next cycle. Treatment should be maintained for at least three consecutive cycles.
Luteal support as part of an Assisted Reproductive Technology (ART) treatment: 1 tablet of Dydrogesterone (Duphaston) 10 three times a day (30 mg daily) starting at the day of oocyte retrieval and continuing for 10 weeks if pregnancy is confirmed.
Hormone replacement therapy: Continuous sequential therapy: An estrogen is dosed continuously and one tablet of 10 mg dydrogesterone is added for the last 14 days of every 28-day cycle, in a sequential manner.
Cyclic therapy: When an estrogen is dosed cyclically with a treatment-free interval, usually 21 days on and 7 days off. One tablet of 10 mg dydrogesterone is added for the last 12 - 14 days of estrogen therapy.
Depending on the clinical response, the dosage can subsequently be adjusted to 20 mg dydrogesterone per day.
There is no relevant use of dydrogesterone before menarche. The safety and efficacy of dydrogesterone in adolescents aged 12-18 years has not been established. Currently available data are described in Adverse Reactions and Pharmacology: Pharmacodynamics under Actions, but no recommendation on a posology can be made.
Method of administration: For oral use.
For administration of higher dosages, the tablets should be taken evenly distributed over the day.
Dysmenorrhea: 10 or 20 mg dydrogesterone per day from day 5 to day 25 of the menstrual cycle.
Endometriosis: 10 to 30 mg dydrogesterone per day from day 5 to day 25 of the cycle or continuously.
Dysfunctional uterine bleeding: When treatment is started to arrest a bleeding episode, 20 or 30 mg dydrogesterone per day is to be given for up to 10 days.
For continuous treatment, 10 or 20 mg dydrogesterone per day should be given during the second half of the menstrual cycle. The starting day and the number of treatment days will depend on the individual cycle length.
Withdrawal bleeding occurs if the endometrium has been adequately primed with either endogenous or exogenous estrogen.
Secondary amenorrhea: 10 or 20 mg dydrogesterone per day, to be given daily for 14 days during the second half of the theoretical menstrual cycle to produce an optimum secretory transformation of an endometrium that has been adequately primed with either endogenous or exogenous estrogen.
Pre-menstrual syndrome: 10 mg dydrogesterone twice daily starting with the second half of the menstrual cycle until the first day of the next cycle. The starting day and the number of treatment days will depend on the individual cycle length.
Irregular cycles: 10 or 20 mg dydrogesterone per day starting with the second half of the menstrual cycle until the first day of the next cycle. The starting day and the number of treatment days will depend on the individual cycle length.
Threatened miscarriage: An initial dose of up to 40 mg dydrogesterone may be given followed by 20 or 30mg per day until symptoms remit.
Habitual miscarriage: 10 mg dydrogesterone twice daily until the twelfth week of pregnancy.
Infertility due to luteal insufficiency: 10 or 20 mg dydrogesterone daily starting with the second half of the menstrual cycle until the first day of the next cycle. Treatment should be maintained for at least three consecutive cycles.
Luteal support as part of an Assisted Reproductive Technology (ART) treatment: 1 tablet of Dydrogesterone (Duphaston) 10 three times a day (30 mg daily) starting at the day of oocyte retrieval and continuing for 10 weeks if pregnancy is confirmed.
Hormone replacement therapy: Continuous sequential therapy: An estrogen is dosed continuously and one tablet of 10 mg dydrogesterone is added for the last 14 days of every 28-day cycle, in a sequential manner.
Cyclic therapy: When an estrogen is dosed cyclically with a treatment-free interval, usually 21 days on and 7 days off. One tablet of 10 mg dydrogesterone is added for the last 12 - 14 days of estrogen therapy.
Depending on the clinical response, the dosage can subsequently be adjusted to 20 mg dydrogesterone per day.
There is no relevant use of dydrogesterone before menarche. The safety and efficacy of dydrogesterone in adolescents aged 12-18 years has not been established. Currently available data are described in Adverse Reactions and Pharmacology: Pharmacodynamics under Actions, but no recommendation on a posology can be made.
Method of administration: For oral use.
For administration of higher dosages, the tablets should be taken evenly distributed over the day.
Overdosage
Limited data are available with regard to overdose in humans. Dydrogesterone was well tolerated after oral dosing (maximum daily dose taken to date in humans 360 mg). There are no specific antidotes and treatment should be symptomatic. This information is also applicable for overdose in children.
Administration
May be taken with or without food.
Contraindications
Known hypersensitivity to the active substance or to any of the excipients.
Known or suspected progestogen dependent neoplasms (e.g. meningioma).
Undiagnosed vaginal bleeding.
Treatment for luteal support as part of an Assisted Reproductive Technology (ART) treatment should be discontinued upon diagnosis of abortion/miscarriage.
Contraindications for the use of estrogens when used in combination with dydrogesterone.
Known or suspected progestogen dependent neoplasms (e.g. meningioma).
Undiagnosed vaginal bleeding.
Treatment for luteal support as part of an Assisted Reproductive Technology (ART) treatment should be discontinued upon diagnosis of abortion/miscarriage.
Contraindications for the use of estrogens when used in combination with dydrogesterone.
Special Precautions
Before initiating dydrogesterone treatment for abnormal bleeding the etiology for the bleeding should be clarified. Breakthrough bleeding and spotting may occur during the first months of treatment. If breakthrough bleeding or spotting appears after some time on therapy, or continues after treatment has been discontinued, the reason should be investigated, which may include endometrial biopsy to exclude endometrial malignancy.
Conditions which need supervision: If any of the following conditions are present, have occurred previously, and/or have been aggravated during pregnancy or previous hormone treatment, the patient should be closely supervised. It should be taken into account that these conditions may recur or be aggravated during treatment with dydrogesterone and ceasing the treatment should be considered: porphyria; depression; abnormal liver function values caused by acute or chronic liver disease.
Other conditions: Patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
The following warnings and precautions apply when using dydrogesterone in combination with estrogens for hormone replacement therapy (HRT): See also the precautions in the product information of the estrogen preparation.
For the treatment of postmenopausal symptoms, HRT should only be initiated for symptoms that adversely affect quality of life. In all cases, a careful appraisal of the risks and benefits should be undertaken at least annually, and HRT should only be continued as long as the benefit outweighs the risk.
Evidence regarding the risks associated with HRT in the treatment of premature menopause is limited. Due to the low level of absolute risk in younger women, however, the balance of benefits and risks for these women may be more favorable than in older women.
Medical examination/follow-up: Before initiating or reinstituting HRT, a complete personal and family medical history should be taken. Physical (including pelvic and breast) examination should be guided by this and by the contraindications and warnings for use. During treatment, periodic check-ups are recommended of a frequency and nature adapted to the individual woman. Women should be advised what changes in their breasts should be reported to their doctor or nurse (see 'Breast cancer' as follows). Investigations, including appropriate imaging tools, e.g., mammography, should be carried out in accordance with currently accepted screening practices, modified to the clinical needs of the individual.
Endometrial hyperplasia and carcinoma: In women with an intact uterus the risk of endometrial hyperplasia and carcinoma is increased when estrogens are administered alone for prolonged periods.
The addition of a progestogen such as dydrogesterone cyclically for at least 12 days per month/28 day cycle or continuous combined estrogen-progestogen therapy in nonhysterectomized women can prevent the excess risk associated with estrogen-only HRT.
Breast cancer: The overall evidence shows an increased risk of breast cancer in women taking combined estrogen-progestogen or estrogen-only HRT, that is dependent on the duration of taking HRT.
Combined estrogen-progestogen therapy: The randomized placebo-controlled trial, Women's Health Initiative study (WHI), and a meta-analysis of prospective epidemiological studies are consistent in finding an increased risk of breast cancer in women taking combined estrogen-progestogen for HRT that becomes apparent after about 3 (1-4) years. Results from a large meta-analysis showed that after stopping treatment, the excess risk will decrease with time and the time needed to return to baseline depends on the duration of prior HRT use. When HRT was taken for more than 5 years, the risk may persist for 10 years or more.
HRT, especially estrogen-progestogen combined treatment, increases the density of mammographic images which may adversely affect the radiological detection of breast cancer.
Ovarian cancer: Ovarian cancer is much rarer than breast cancer. Epidemiological evidence from a large meta-analysis suggests a slightly increased risk in women taking estrogen-only or combined estrogen-progestogen HRT, which becomes apparent within 5 years of use and diminishes over time after stopping. Some other studies including the WHI trial suggest that use of combined HRTs may be associated a similar, or slightly smaller, risk.
Venous thrombo-embolism: HRT is associated with a 1.3-3-fold risk of developing venous thromboembolism (VTE), i.e., deep vein thrombosis or pulmonary embolism. The occurrence of such an event is more likely in the first year of HRT than later. Patients with known thrombophilic states have an increased risk of VTE and HRT may add to this risk. HRT is therefore contraindicated in these patients.
Generally recognized risk factors for VTE include, use of estrogens, older age, major surgery, prolonged immobilization, obesity (BMI > 30 kg/m2), pregnancy/postpartum period, systemic lupus erythematosus (SLE), and cancer. There is no consensus about the possible role of varicose veins in VTE.
As in all postoperative patients, prophylactic measures need be considered to prevent VTE following surgery. If prolonged immobilization is to follow elective surgery temporarily stopping HRT 4 to 6 weeks earlier is recommended. Treatment should not be restarted until the woman is completely mobilized. In women with no personal history of VTE but with a first degree relative with a history of thrombosis at young age, screening may be offered after careful counseling regarding its limitations (only a proportion of thrombophilic defects are identified by screening).
If a thrombophilic defect is identified which segregates with thrombosis in family members or if the defect is 'severe' (e.g., antithrombin, protein S, or protein C deficiencies or a combination of defects) HRT is contraindicated.
Women already on chronic anticoagulant treatment require careful consideration of the benefit risk of use of HRT.
If VTE develops after initiating therapy, the drug should be discontinued. Patients should be told to contact their doctors immediately when they are aware of a potential thromboembolic symptom (e.g., painful swelling of a leg, sudden pain in the chest, dyspnea).
Coronary artery disease (CAD): There is no evidence from randomized controlled trials of protection against myocardial infarction in women with or without existing CAD who received combined estrogen-progestogen or estrogen-only HRT. Combined estrogen-progestogen therapy: The relative risk of CAD during use of combined estrogen-progestogen HRT is slightly increased. As the baseline absolute risk of CAD is strongly dependent on age, the number of extra cases of CAD due to estrogen-progestogen use is very low in healthy women close to menopause but will rise with more advanced age.
Ischemic stroke: Combined estrogen-progestogen and estrogen-only therapy are associated with an up to 1.5-fold increase in risk of ischemic stroke. The relative risk does not change with age or time since menopause. However, as the baseline risk of stroke is strongly age-dependent, the overall risk of stroke in women who use HRT will increase with age.
Excipients: This medicinal product contains Lactose monohydrate.
Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Effects on Ability to Drive and Use Machines: Dydrogesterone has minor influence on the ability to drive and use machines.
Infrequently, dydrogesterone may cause mild somnolence and/or dizziness, especially within the first few hours after intake. Therefore, care should be taken when driving or using machines.
Conditions which need supervision: If any of the following conditions are present, have occurred previously, and/or have been aggravated during pregnancy or previous hormone treatment, the patient should be closely supervised. It should be taken into account that these conditions may recur or be aggravated during treatment with dydrogesterone and ceasing the treatment should be considered: porphyria; depression; abnormal liver function values caused by acute or chronic liver disease.
Other conditions: Patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
The following warnings and precautions apply when using dydrogesterone in combination with estrogens for hormone replacement therapy (HRT): See also the precautions in the product information of the estrogen preparation.
For the treatment of postmenopausal symptoms, HRT should only be initiated for symptoms that adversely affect quality of life. In all cases, a careful appraisal of the risks and benefits should be undertaken at least annually, and HRT should only be continued as long as the benefit outweighs the risk.
Evidence regarding the risks associated with HRT in the treatment of premature menopause is limited. Due to the low level of absolute risk in younger women, however, the balance of benefits and risks for these women may be more favorable than in older women.
Medical examination/follow-up: Before initiating or reinstituting HRT, a complete personal and family medical history should be taken. Physical (including pelvic and breast) examination should be guided by this and by the contraindications and warnings for use. During treatment, periodic check-ups are recommended of a frequency and nature adapted to the individual woman. Women should be advised what changes in their breasts should be reported to their doctor or nurse (see 'Breast cancer' as follows). Investigations, including appropriate imaging tools, e.g., mammography, should be carried out in accordance with currently accepted screening practices, modified to the clinical needs of the individual.
Endometrial hyperplasia and carcinoma: In women with an intact uterus the risk of endometrial hyperplasia and carcinoma is increased when estrogens are administered alone for prolonged periods.
The addition of a progestogen such as dydrogesterone cyclically for at least 12 days per month/28 day cycle or continuous combined estrogen-progestogen therapy in nonhysterectomized women can prevent the excess risk associated with estrogen-only HRT.
Breast cancer: The overall evidence shows an increased risk of breast cancer in women taking combined estrogen-progestogen or estrogen-only HRT, that is dependent on the duration of taking HRT.
Combined estrogen-progestogen therapy: The randomized placebo-controlled trial, Women's Health Initiative study (WHI), and a meta-analysis of prospective epidemiological studies are consistent in finding an increased risk of breast cancer in women taking combined estrogen-progestogen for HRT that becomes apparent after about 3 (1-4) years. Results from a large meta-analysis showed that after stopping treatment, the excess risk will decrease with time and the time needed to return to baseline depends on the duration of prior HRT use. When HRT was taken for more than 5 years, the risk may persist for 10 years or more.
HRT, especially estrogen-progestogen combined treatment, increases the density of mammographic images which may adversely affect the radiological detection of breast cancer.
Ovarian cancer: Ovarian cancer is much rarer than breast cancer. Epidemiological evidence from a large meta-analysis suggests a slightly increased risk in women taking estrogen-only or combined estrogen-progestogen HRT, which becomes apparent within 5 years of use and diminishes over time after stopping. Some other studies including the WHI trial suggest that use of combined HRTs may be associated a similar, or slightly smaller, risk.
Venous thrombo-embolism: HRT is associated with a 1.3-3-fold risk of developing venous thromboembolism (VTE), i.e., deep vein thrombosis or pulmonary embolism. The occurrence of such an event is more likely in the first year of HRT than later. Patients with known thrombophilic states have an increased risk of VTE and HRT may add to this risk. HRT is therefore contraindicated in these patients.
Generally recognized risk factors for VTE include, use of estrogens, older age, major surgery, prolonged immobilization, obesity (BMI > 30 kg/m2), pregnancy/postpartum period, systemic lupus erythematosus (SLE), and cancer. There is no consensus about the possible role of varicose veins in VTE.
As in all postoperative patients, prophylactic measures need be considered to prevent VTE following surgery. If prolonged immobilization is to follow elective surgery temporarily stopping HRT 4 to 6 weeks earlier is recommended. Treatment should not be restarted until the woman is completely mobilized. In women with no personal history of VTE but with a first degree relative with a history of thrombosis at young age, screening may be offered after careful counseling regarding its limitations (only a proportion of thrombophilic defects are identified by screening).
If a thrombophilic defect is identified which segregates with thrombosis in family members or if the defect is 'severe' (e.g., antithrombin, protein S, or protein C deficiencies or a combination of defects) HRT is contraindicated.
Women already on chronic anticoagulant treatment require careful consideration of the benefit risk of use of HRT.
If VTE develops after initiating therapy, the drug should be discontinued. Patients should be told to contact their doctors immediately when they are aware of a potential thromboembolic symptom (e.g., painful swelling of a leg, sudden pain in the chest, dyspnea).
Coronary artery disease (CAD): There is no evidence from randomized controlled trials of protection against myocardial infarction in women with or without existing CAD who received combined estrogen-progestogen or estrogen-only HRT. Combined estrogen-progestogen therapy: The relative risk of CAD during use of combined estrogen-progestogen HRT is slightly increased. As the baseline absolute risk of CAD is strongly dependent on age, the number of extra cases of CAD due to estrogen-progestogen use is very low in healthy women close to menopause but will rise with more advanced age.
Ischemic stroke: Combined estrogen-progestogen and estrogen-only therapy are associated with an up to 1.5-fold increase in risk of ischemic stroke. The relative risk does not change with age or time since menopause. However, as the baseline risk of stroke is strongly age-dependent, the overall risk of stroke in women who use HRT will increase with age.
Excipients: This medicinal product contains Lactose monohydrate.
Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Effects on Ability to Drive and Use Machines: Dydrogesterone has minor influence on the ability to drive and use machines.
Infrequently, dydrogesterone may cause mild somnolence and/or dizziness, especially within the first few hours after intake. Therefore, care should be taken when driving or using machines.
Use In Pregnancy & Lactation
Pregnancy: It is estimated that more than 10 million pregnancies have been exposed to dydrogesterone. So far there were no indications of a harmful effect of dydrogesterone use during pregnancy. Some progestogens have been reported in the literature to be associated with an increased risk of hypospadias. However due to confounding factors during pregnancy, no definitive conclusion can be drawn regarding the contribution of progestogens to hypospadias. Clinical studies, where a limited number of women were treated with dydrogesterone early in pregnancy, have not shown any increase in risk. No other epidemiological data are hitherto available.
Effects in non-clinical embryo-fetal and post-natal development studies were in line with the pharmacological profile. Untoward effects occurred only at exposures which exceeded the maximum human exposure considerably, indicating little relevance to clinical use (see Pharmacology: Toxicology: Preclinical Safety Data under Actions). Dydrogesterone can be used during pregnancy if clearly indicated.
Breastfeeding: No data exist on excretion of dydrogesterone in mother's milk. Experience with other progestogens indicates that progestogens and the metabolites pass to mother's milk in small quantities. Whether there is a risk to the child is not known. Therefore, dydrogesterone should not be used during the lactation period.
Fertility: There is no evidence that dydrogesterone decreases fertility at therapeutic dose.
Effects in non-clinical embryo-fetal and post-natal development studies were in line with the pharmacological profile. Untoward effects occurred only at exposures which exceeded the maximum human exposure considerably, indicating little relevance to clinical use (see Pharmacology: Toxicology: Preclinical Safety Data under Actions). Dydrogesterone can be used during pregnancy if clearly indicated.
Breastfeeding: No data exist on excretion of dydrogesterone in mother's milk. Experience with other progestogens indicates that progestogens and the metabolites pass to mother's milk in small quantities. Whether there is a risk to the child is not known. Therefore, dydrogesterone should not be used during the lactation period.
Fertility: There is no evidence that dydrogesterone decreases fertility at therapeutic dose.
Adverse Reactions
The most commonly reported adverse drug reactions of patients treated with dydrogesterone in clinical trials of indications without estrogen treatment are vaginal hemorrhage, migraines/headache, nausea, vomiting, abdominal pain, menstrual disorders and breast pain/tenderness.
The following undesirable effects have been observed with the frequencies indicated as follows during clinical trials using dydrogesterone (n=3483) in indications without estrogen treatment, in two company sponsored interventional clinical trials in luteal support as part of an ART treatment using dydrogesterone (n=1036) and from spontaneous reporting. Frequencies are based on the most conservative approach. (See table.)
The following undesirable effects have been observed with the frequencies indicated as follows during clinical trials using dydrogesterone (n=3483) in indications without estrogen treatment, in two company sponsored interventional clinical trials in luteal support as part of an ART treatment using dydrogesterone (n=1036) and from spontaneous reporting. Frequencies are based on the most conservative approach. (See table.)
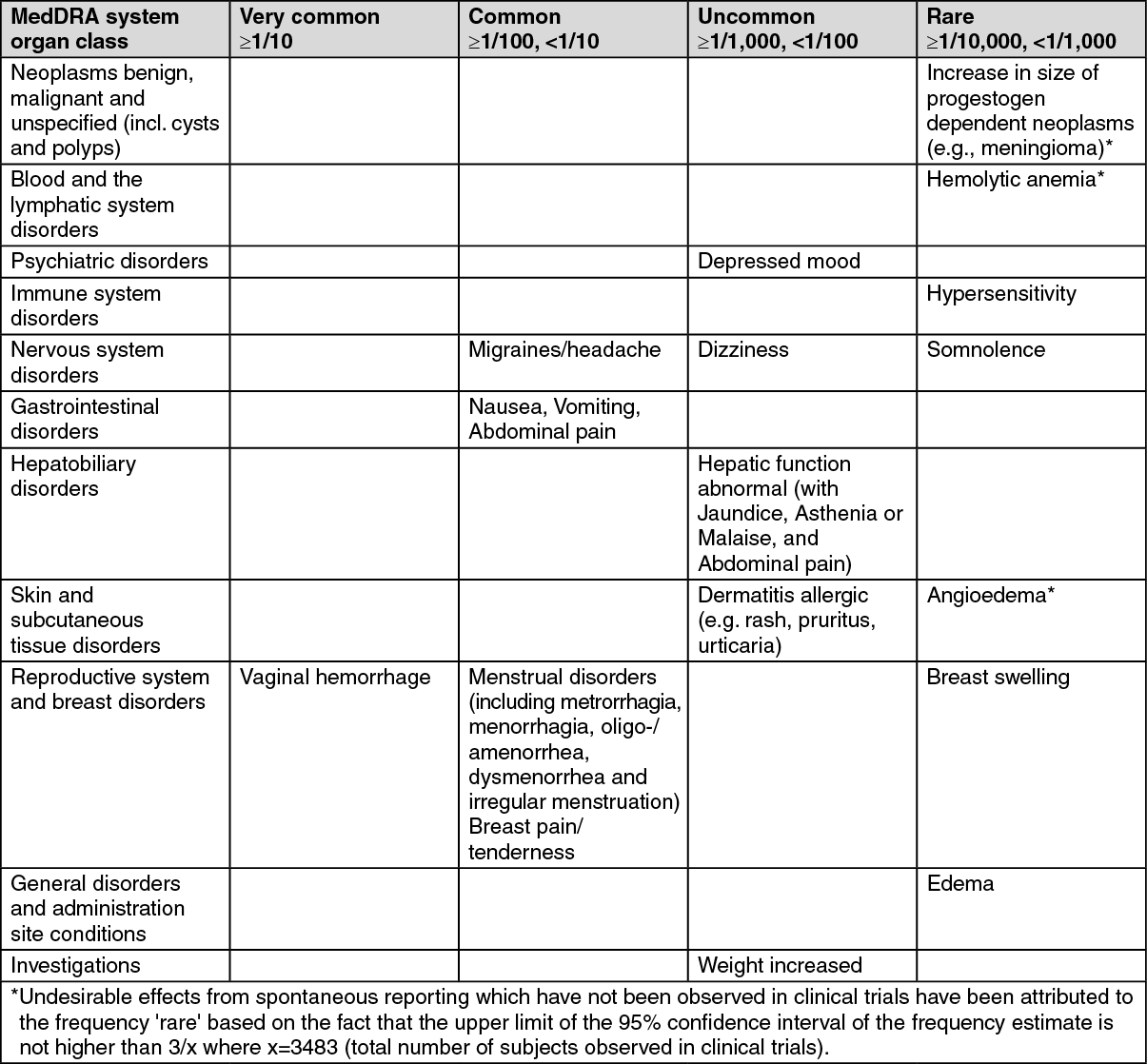
Undesirable effects in adolescent population: Based on spontaneous reports and limited clinical trial data, the adverse reaction profile in adolescents is expected to be similar to that seen in adults.
Undesirable effects that are associated with an estrogen-progestogen treatment (see also Precautions and the product information of the estrogen preparation): Breast cancer, endometrial hyperplasia, endometrial carcinoma, ovarian cancer; Venous thromboembolism; Myocardial infarction, coronary artery disease, ischemic stroke.
Undesirable effects that are associated with an estrogen-progestogen treatment (see also Precautions and the product information of the estrogen preparation): Breast cancer, endometrial hyperplasia, endometrial carcinoma, ovarian cancer; Venous thromboembolism; Myocardial infarction, coronary artery disease, ischemic stroke.
Drug Interactions
In vitro data show that the major metabolic pathway generating the main pharmacologically active metabolite 20α dihydrodydrogesterone (DHD) is catalyzed by aldo-keto reductase 1C (AKR 1C) in human cytosol. Next to the cytosolic metabolism there are metabolic transformations by cytochrome P450 iso-enzymes (CYPs), nearly exclusively via CYP3A4, resulting in several minor metabolites. The main active metabolite DHD is substrate for metabolic transformation by CYP3A4. Therefore, the metabolism of dydrogesterone and DHD may be increased by concomitant use of substances known to induce CYP enzymes such as anticonvulsants (e.g., phenobarbital, phenytoin, carbamazepine), anti-infectives (e.g., rifampicin, rifabutin, nevirapine, efavirenz) and herbal preparations containing e.g. St John's Wort (Hypericum perforatum), sage, or gingko biloba. Ritonavir and nelfinavir, although known as strong cytochrome enzyme inhibitors, by contrast exhibit enzyme-inducing properties when used concomitantly with steroid hormones.
Clinically, an increased metabolism of dydrogesterone may lead to a decreased effect.
In vitro studies have shown that dydrogesterone and DHD do not inhibit or induce CYP drug metabolizing enzymes at clinically relevant concentrations.
Clinically, an increased metabolism of dydrogesterone may lead to a decreased effect.
In vitro studies have shown that dydrogesterone and DHD do not inhibit or induce CYP drug metabolizing enzymes at clinically relevant concentrations.
Caution For Usage
Incompatibilities: Not applicable.
Storage
Store at temperatures not exceeding 30°C.
Shelf-life: 60 months.
Shelf-life: 60 months.
Action
Pharmacotherapeutic group: Genito-urinary system and sex hormones. ATC code: G03DB01.
Pharmacology: Pharmacodynamics: Mechanism of action: Dydrogesterone is an orally-active progestogen which produces a complete secretory endometrium in an estrogen-primed uterus thereby providing protection against the increased risk for endometrium hyperplasia and/or carcinogenesis induced by estrogens. It is indicated in all cases of endogenous progesterone deficiency. Dydrogesterone has no estrogenic, no androgenic, no thermogenic, no anabolic and no corticoid activity.
Clinical efficacy and safety: Lotus I and Lotus II clinical study(s) confirmed the following: A Double-Blind, Double-Dummy, Randomized, Two-arm, Multicenter Study Comparing the Efficacy, Safety, and Tolerability of Oral Dydrogesterone 30 mg daily versus Intravaginal Micronized Progesterone Capsules 600 mg daily for Luteal Support in In-Vitro Fertilization (LOTUS I).
A Randomized, Open-label, Two-arm, Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg daily versus Crinone 8% intravaginal progesterone gel 90 mg daily for Luteal Support in In Vitro Fertilization (LOTUS II).
The primary objective of non-inferiority of oral dydrogesterone compared to intravaginal micronized progesterone in terms of the presence of fetal heartbeats at 12 weeks' gestation (10 weeks pregnancy) was achieved.
In the studied patient population, pregnancy rates at 12 weeks' gestation (pregnancy week 10) were 37.6% and 33.1% (LOTUS I) and 36.7% and 34.7% (LOTUS II) for oral dydrogesterone and micronized vaginal progesterone respectively. The difference in the pregnancy rate between the two groups was 4.7 (95% CI, -1.2; 10.6) (LOTUS I) and 2.0 (95% CI, -4.0; 8.0) (LOTUS II).
Within the safety sample of 1029 subjects (LOTUS I) and 1030 subjects (LOTUS II) with at least one dose of study medication administered, the incidence of the most frequently reported TEAE was similar between the two treatment groups.
Due to the nature of the studied patient population/indication a number of early abortions/miscarriages are expected; especially until 12 weeks' gestation (pregnancy week 10) as the expected pregnancy rate at this time point is about 35%.
The safety profile observed in both LOTUS studies is as expected taking into account the well-established safety profile of dydrogesterone and the treatment population/indication.
Adolescent population: Limited clinical trial data indicate that dydrogesterone is efficacious in relieving symptoms of dysmenorrhea, premenstrual syndrome, dysfunctional uterine bleeding and irregular cycles in the population of patients younger than 18 years of age in a similar manner as in the adult population.
Pharmacokinetics: Absorption: Following oral administration, dydrogesterone film-coated tablets, is rapidly absorbed. Maximum plasma concentrations of about 3.2 ng/ml and 57 ng/ml are attained between 0.5 and 1.5 hours after dosing for the parent drug dydrogesterone and its active metabolite 20α-dihydrodydrogesterone (DHD) respectively. The total drug exposures across time (AUC) are about 9.1 and 220 ng.hr/ml for Dydrogesterone and DHD respectively.
After a single dose, food delays the peak plasma concentration of dydrogesterone with approximately 1 hour, resulting in approximately 20% lower dydrogesterone peak plasma concentrations without affecting the extent of exposure to dydrogesterone and DHD.
The observed effect of concomitant food intake on the peak plasma concentration of dydrogesterone is considered not clinically relevant. Therefore, Dydrogesterone (Duphaston) film-coated tablets can be taken without regards to food.
Distribution: After oral administration of dydrogesterone the apparent volume of distribution is large, being approximately 22000 L. Dydrogesterone and DHD are more than 90% bound to plasma proteins.
Metabolism: Following oral administration, dydrogesterone is rapidly metabolized to DHD. The levels of the main active metabolite DHD peak at similar times as Dydrogesterone.
The plasma levels of DHD are substantially higher as compared to the parent drug. The AUC and Cmax ratios of DHD to dydrogesterone are approximately 25 and 20, respectively. The mean terminal elimination half-lives of both dydrogesterone and DHD is about 15 hours.
A common feature of all metabolites characterised is the retention of the 4,6 diene-3-one configuration of the parent compound and the absence of 17α-hydroxylation. This explains the lack of estrogenic and androgenic effects of dydrogesterone.
Elimination: After oral administration of labelled dydrogesterone, on average 63% of the dose is excreted into the urine. The apparent total body clearance of dydrogesterone from plasma is high at approximately 20 L/min.
Within 72 hours excretion is complete. DHD is present in the urine predominantly as the glucuronic acid conjugate.
Dose and time dependencies: The single and multiple dose pharmacokinetics are linear in the oral dose range 2.5 to 10 mg. Comparison of the single and multiple dose kinetics shows that the pharmacokinetics of dydrogesterone and DHD are not changed as a result of repeated dosing. Steady state conditions are generally reached after 3 days of treatment.
Toxicology: Preclinical Safety Data: Non-clinical data obtained from conventional studies on single and repeated dose toxicity, genotoxicity and carcinogenic potential reveal no special hazard for humans.
Reproduction toxicity studies in rats have shown an increased incidence of prominent nipples (between day 11 and day 19 of age) and of hypospadias in the male offspring at high dosages not comparable to human exposure. The actual risk of hypospadias in humans cannot be determined in animal studies due to major species differences in metabolism between rats and humans (see also Use in Pregnancy & Lactation).
Limited animal safety data suggest that dydrogesterone has prolongating effects on parturition, which is consistent with its progestogenic activity.
Pharmacology: Pharmacodynamics: Mechanism of action: Dydrogesterone is an orally-active progestogen which produces a complete secretory endometrium in an estrogen-primed uterus thereby providing protection against the increased risk for endometrium hyperplasia and/or carcinogenesis induced by estrogens. It is indicated in all cases of endogenous progesterone deficiency. Dydrogesterone has no estrogenic, no androgenic, no thermogenic, no anabolic and no corticoid activity.
Clinical efficacy and safety: Lotus I and Lotus II clinical study(s) confirmed the following: A Double-Blind, Double-Dummy, Randomized, Two-arm, Multicenter Study Comparing the Efficacy, Safety, and Tolerability of Oral Dydrogesterone 30 mg daily versus Intravaginal Micronized Progesterone Capsules 600 mg daily for Luteal Support in In-Vitro Fertilization (LOTUS I).
A Randomized, Open-label, Two-arm, Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg daily versus Crinone 8% intravaginal progesterone gel 90 mg daily for Luteal Support in In Vitro Fertilization (LOTUS II).
The primary objective of non-inferiority of oral dydrogesterone compared to intravaginal micronized progesterone in terms of the presence of fetal heartbeats at 12 weeks' gestation (10 weeks pregnancy) was achieved.
In the studied patient population, pregnancy rates at 12 weeks' gestation (pregnancy week 10) were 37.6% and 33.1% (LOTUS I) and 36.7% and 34.7% (LOTUS II) for oral dydrogesterone and micronized vaginal progesterone respectively. The difference in the pregnancy rate between the two groups was 4.7 (95% CI, -1.2; 10.6) (LOTUS I) and 2.0 (95% CI, -4.0; 8.0) (LOTUS II).
Within the safety sample of 1029 subjects (LOTUS I) and 1030 subjects (LOTUS II) with at least one dose of study medication administered, the incidence of the most frequently reported TEAE was similar between the two treatment groups.
Due to the nature of the studied patient population/indication a number of early abortions/miscarriages are expected; especially until 12 weeks' gestation (pregnancy week 10) as the expected pregnancy rate at this time point is about 35%.
The safety profile observed in both LOTUS studies is as expected taking into account the well-established safety profile of dydrogesterone and the treatment population/indication.
Adolescent population: Limited clinical trial data indicate that dydrogesterone is efficacious in relieving symptoms of dysmenorrhea, premenstrual syndrome, dysfunctional uterine bleeding and irregular cycles in the population of patients younger than 18 years of age in a similar manner as in the adult population.
Pharmacokinetics: Absorption: Following oral administration, dydrogesterone film-coated tablets, is rapidly absorbed. Maximum plasma concentrations of about 3.2 ng/ml and 57 ng/ml are attained between 0.5 and 1.5 hours after dosing for the parent drug dydrogesterone and its active metabolite 20α-dihydrodydrogesterone (DHD) respectively. The total drug exposures across time (AUC) are about 9.1 and 220 ng.hr/ml for Dydrogesterone and DHD respectively.
After a single dose, food delays the peak plasma concentration of dydrogesterone with approximately 1 hour, resulting in approximately 20% lower dydrogesterone peak plasma concentrations without affecting the extent of exposure to dydrogesterone and DHD.
The observed effect of concomitant food intake on the peak plasma concentration of dydrogesterone is considered not clinically relevant. Therefore, Dydrogesterone (Duphaston) film-coated tablets can be taken without regards to food.
Distribution: After oral administration of dydrogesterone the apparent volume of distribution is large, being approximately 22000 L. Dydrogesterone and DHD are more than 90% bound to plasma proteins.
Metabolism: Following oral administration, dydrogesterone is rapidly metabolized to DHD. The levels of the main active metabolite DHD peak at similar times as Dydrogesterone.
The plasma levels of DHD are substantially higher as compared to the parent drug. The AUC and Cmax ratios of DHD to dydrogesterone are approximately 25 and 20, respectively. The mean terminal elimination half-lives of both dydrogesterone and DHD is about 15 hours.
A common feature of all metabolites characterised is the retention of the 4,6 diene-3-one configuration of the parent compound and the absence of 17α-hydroxylation. This explains the lack of estrogenic and androgenic effects of dydrogesterone.
Elimination: After oral administration of labelled dydrogesterone, on average 63% of the dose is excreted into the urine. The apparent total body clearance of dydrogesterone from plasma is high at approximately 20 L/min.
Within 72 hours excretion is complete. DHD is present in the urine predominantly as the glucuronic acid conjugate.
Dose and time dependencies: The single and multiple dose pharmacokinetics are linear in the oral dose range 2.5 to 10 mg. Comparison of the single and multiple dose kinetics shows that the pharmacokinetics of dydrogesterone and DHD are not changed as a result of repeated dosing. Steady state conditions are generally reached after 3 days of treatment.
Toxicology: Preclinical Safety Data: Non-clinical data obtained from conventional studies on single and repeated dose toxicity, genotoxicity and carcinogenic potential reveal no special hazard for humans.
Reproduction toxicity studies in rats have shown an increased incidence of prominent nipples (between day 11 and day 19 of age) and of hypospadias in the male offspring at high dosages not comparable to human exposure. The actual risk of hypospadias in humans cannot be determined in animal studies due to major species differences in metabolism between rats and humans (see also Use in Pregnancy & Lactation).
Limited animal safety data suggest that dydrogesterone has prolongating effects on parturition, which is consistent with its progestogenic activity.
MedsGo Class
Oestrogens, Progesterones & Related Synthetic Drugs
Features
Dosage
10mg
Ingredients
- Dydrogesterone
Packaging
Tablet 1's
Generic Name
Dydrogesterone
Registration Number
DRP-221
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions



