Indications/Uses
Rabeprazole sodium (PARIET) tablets are indicated for the treatment of: Active duodenal ulcer; Active benign gastric ulcer; Anastomotic ulcer; Erosive or ulcerative gastroesophageal reflux disease (GERD); Gastro-esophageal reflux disease long-term management (GERD maintenance); Symptomatic treatment of moderate to very severe gastro-esophageal reflux disease (symptomatic GERD); Zollinger-Ellison Syndrome and other pathological hypersecretory conditions; Prevention of gastric and duodenal ulcer recurrences associated with low-dose aspirin therapy; In combination with appropriate antibacterial therapeutic regimens for the eradication of Helicobacter pylori in patients with peptic ulcer disease. See Dosage & Administration.
Dosage/Direction for Use
Adults/Elderly: Active Duodenal Ulcer, Active Benign Gastric Ulcer and Anastomotic Ulcer: The recommended oral dose for both active duodenal ulcer, active benign gastric ulcer and anastomotic ulcer is 10 mg or 20 mg to be taken once daily in the morning.
Most patients with active duodenal ulcer heal within four weeks. However a few patients may require an additional four weeks of therapy to achieve healing. Most patients with active benign gastric ulcer heal within six weeks. However, again a few patients may require an additional six weeks of therapy to achieve healing.
Erosive or Ulcerative Gastro-Esophageal Reflux Disease (GERD): The recommended oral dose for this condition is 10 mg or 20 mg to be taken once daily for four to eight weeks. Doses of 10 mg or 20 mg twice daily may be administered orally for another eight weeks in reflux esophagitis patients who are not responding to the usual dose of proton pump inhibitors. However, a dose of 20 mg twice daily should only be administered to patients with severe mucosa injury.
Gastro-Esophageal Reflux Disease Long-term Management (GERD maintenance): For long-term management, a maintenance dose of Rabeprazole sodium (PARIET) 10 mg or 20 mg once daily can be used depending upon patient response. For the maintenance therapy when proton pump inhibitor treatment is ineffective, dose of 10 mg twice daily may be administered orally.
Symptomatic Treatment of Moderate to Very Severe Gastro-Esophageal Reflux Disease (symptomatic GERD): 10 mg once daily in patients without esophagitis. If symptom control has not been achieved during four weeks, the patient should be further investigated. Once symptoms have resolved, subsequent symptom control can be achieved using an on-demand regimen taking 10 mg once daily when needed.
Zollinger-Ellison Syndrome and Other Pathological Hypersecretory Conditions: The dose varies with the individual patient. A starting dose of 60 mg daily, and doses of up to 100 mg once daily, or 60 mg twice daily have been used. Some patients may require divided doses. Dosing should continue for as long as clinically necessary. Some patients with Zollinger-Ellison Syndrome have been treated continuously for up to one year.
For the prevention of gastric and duodenal ulcer recurrences associated with low-dose aspirin therapy, the usual dosage for adults is 10 mg administered orally once a day.
Eradication of H. pylori: Patients with H. pylori infection should be treated with eradication therapy. The following combination given for 7 days is recommended: Rabeprazole sodium (PARIET) 10 mg or 20 mg twice daily + clarithromycin 500 mg twice daily and amoxicillin 1 g twice daily.
For indications requiring once daily treatment, Rabeprazole sodium (PARIET) tablets should be taken in the morning, before eating; and although neither the time of day nor food intake was shown to have any effect on rabeprazole sodium activity, this regimen will facilitate treatment compliance.
Patients should be cautioned that the Rabeprazole sodium (PARIET) tablets should not be chewed or crushed, but should be swallowed whole.
Renal and hepatic impairment: No dosage adjustment is necessary for patients with renal or hepatic impairment.
See Precautions for Use of Rabeprazole sodium (PARIET) in the treatment of patients with severe hepatic impairment.
Children: Safety and effectiveness of Rabeprazole sodium (PARIET) for the short-term (up to eight weeks) treatment of GERD in adolescents 12 years of age and above is supported by extrapolation of results from adequate and well-controlled studies that supported the effectiveness of Rabeprazole sodium (PARIET) for adults; safety and pharmacokinetic studies performed in adolescent patients. The recommended oral dose for adolescents 12 years of age and above is 20 mg once daily for up to eight weeks.
Rabeprazole sodium (PARIET) is not recommended for the treatment of GERD in children <12 years of age, as there is no experience of its use in this group. The safety and effectiveness of rabeprazole sodium for other uses have not been established in pediatric patients.
Most patients with active duodenal ulcer heal within four weeks. However a few patients may require an additional four weeks of therapy to achieve healing. Most patients with active benign gastric ulcer heal within six weeks. However, again a few patients may require an additional six weeks of therapy to achieve healing.
Erosive or Ulcerative Gastro-Esophageal Reflux Disease (GERD): The recommended oral dose for this condition is 10 mg or 20 mg to be taken once daily for four to eight weeks. Doses of 10 mg or 20 mg twice daily may be administered orally for another eight weeks in reflux esophagitis patients who are not responding to the usual dose of proton pump inhibitors. However, a dose of 20 mg twice daily should only be administered to patients with severe mucosa injury.
Gastro-Esophageal Reflux Disease Long-term Management (GERD maintenance): For long-term management, a maintenance dose of Rabeprazole sodium (PARIET) 10 mg or 20 mg once daily can be used depending upon patient response. For the maintenance therapy when proton pump inhibitor treatment is ineffective, dose of 10 mg twice daily may be administered orally.
Symptomatic Treatment of Moderate to Very Severe Gastro-Esophageal Reflux Disease (symptomatic GERD): 10 mg once daily in patients without esophagitis. If symptom control has not been achieved during four weeks, the patient should be further investigated. Once symptoms have resolved, subsequent symptom control can be achieved using an on-demand regimen taking 10 mg once daily when needed.
Zollinger-Ellison Syndrome and Other Pathological Hypersecretory Conditions: The dose varies with the individual patient. A starting dose of 60 mg daily, and doses of up to 100 mg once daily, or 60 mg twice daily have been used. Some patients may require divided doses. Dosing should continue for as long as clinically necessary. Some patients with Zollinger-Ellison Syndrome have been treated continuously for up to one year.
For the prevention of gastric and duodenal ulcer recurrences associated with low-dose aspirin therapy, the usual dosage for adults is 10 mg administered orally once a day.
Eradication of H. pylori: Patients with H. pylori infection should be treated with eradication therapy. The following combination given for 7 days is recommended: Rabeprazole sodium (PARIET) 10 mg or 20 mg twice daily + clarithromycin 500 mg twice daily and amoxicillin 1 g twice daily.
For indications requiring once daily treatment, Rabeprazole sodium (PARIET) tablets should be taken in the morning, before eating; and although neither the time of day nor food intake was shown to have any effect on rabeprazole sodium activity, this regimen will facilitate treatment compliance.
Patients should be cautioned that the Rabeprazole sodium (PARIET) tablets should not be chewed or crushed, but should be swallowed whole.
Renal and hepatic impairment: No dosage adjustment is necessary for patients with renal or hepatic impairment.
See Precautions for Use of Rabeprazole sodium (PARIET) in the treatment of patients with severe hepatic impairment.
Children: Safety and effectiveness of Rabeprazole sodium (PARIET) for the short-term (up to eight weeks) treatment of GERD in adolescents 12 years of age and above is supported by extrapolation of results from adequate and well-controlled studies that supported the effectiveness of Rabeprazole sodium (PARIET) for adults; safety and pharmacokinetic studies performed in adolescent patients. The recommended oral dose for adolescents 12 years of age and above is 20 mg once daily for up to eight weeks.
Rabeprazole sodium (PARIET) is not recommended for the treatment of GERD in children <12 years of age, as there is no experience of its use in this group. The safety and effectiveness of rabeprazole sodium for other uses have not been established in pediatric patients.
Overdosage
Experience to date with deliberate or accidental overdose is limited. The maximum established exposure has not exceeded 60 mg twice daily or 160 mg once daily. Effects are generally minimal, representative of the known adverse event profile and reversible without further medical intervention. No specific antidote is known. Rabeprazole sodium is extensively protein bound and is, therefore, not dialysable. As in any case of overdose, treatment should be symptomatic and general supportive measures should be utilized.
Administration
May be taken with or without food: For once daily dosing, take tab in the morning before eating. Swallow whole, do not chew/crush.
Contraindications
Rabeprazole sodium (PARIET) is contraindicated in patients with known hypersensitivity to rabeprazole sodium, substituted benzimidazoles or to any excipient used in the formulation. Rabeprazole sodium (PARIET) is contraindicated in pregnancy and during breast feeding.
Special Precautions
Symptomatic response to therapy with rabeprazole sodium does not preclude the presence of gastric or esophageal malignancy therefore the possibility of malignancy should be excluded prior to commencing treatment with Rabeprazole sodium (PARIET).
Patients on long-term treatment (particularly those treated for more than a year) should be kept under regular surveillance.
No evidence of significant drug related safety problems was seen in a study of patients with mild to moderate hepatic impairment versus normal age and sex matched controls. However because there are no clinical data on the use of Rabeprazole sodium (PARIET) in the treatment of patients with severe hepatic dysfunction the prescriber is advised to exercise caution when treatment with Rabeprazole sodium (PARIET) is first initiated in such patients. The exposure to rabeprazole sodium (AUC) in patients with significant hepatic dysfunction is approximately two-fold that of healthy patients.
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically. See Adverse Reactions.
Fractures: Observational studies suggest that proton pump inhibitor (PPI) therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, and long-term PPI therapy (a year or longer).
Concomitant use of Rabeprazole with Methotrexate: Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients.
Clostridium difficile: Treatment with proton pump inhibitors may possibly increase the risk of gastrointestinal infections such as Clostridium difficile.
Subacute cutaneous lupus erythematosus: Subacute cutaneous lupus erythematosus (SCLE) has been reported with the use of Proton Pump Inhibitors (PPIs). If lesions occur, especially in sun-exposed areas of the skin, and if accompanied by arthralgia, the patient should seek medical help promptly and the health care professional should consider stopping rabeprazole. The occurrence of SCLE with previous PPI treatment may increase the risk of SCLE with other PPIs.
Fundic Gland Polyps: Long term PPI use, including rabeprazole, appears to be associated with an increased risk of fundic gland polyps. Most fundic gland polyps are asymptomatic. Patients with large or ulcerated polyps may be at risk of gastrointestinal bleeding or small intestinal blockage. Use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
Effects on ability to Drive and use Machines: Based on pharmacodynamic properties and the adverse events profile, it is unlikely that Rabeprazole sodium (PARIET) would cause an impairment of driving performance or compromise the ability to use machinery. If however, alertness is impaired due to somnolence, it is recommended that driving and operating complex machinery be avoided.
Patients on long-term treatment (particularly those treated for more than a year) should be kept under regular surveillance.
No evidence of significant drug related safety problems was seen in a study of patients with mild to moderate hepatic impairment versus normal age and sex matched controls. However because there are no clinical data on the use of Rabeprazole sodium (PARIET) in the treatment of patients with severe hepatic dysfunction the prescriber is advised to exercise caution when treatment with Rabeprazole sodium (PARIET) is first initiated in such patients. The exposure to rabeprazole sodium (AUC) in patients with significant hepatic dysfunction is approximately two-fold that of healthy patients.
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically. See Adverse Reactions.
Fractures: Observational studies suggest that proton pump inhibitor (PPI) therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, and long-term PPI therapy (a year or longer).
Concomitant use of Rabeprazole with Methotrexate: Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients.
Clostridium difficile: Treatment with proton pump inhibitors may possibly increase the risk of gastrointestinal infections such as Clostridium difficile.
Subacute cutaneous lupus erythematosus: Subacute cutaneous lupus erythematosus (SCLE) has been reported with the use of Proton Pump Inhibitors (PPIs). If lesions occur, especially in sun-exposed areas of the skin, and if accompanied by arthralgia, the patient should seek medical help promptly and the health care professional should consider stopping rabeprazole. The occurrence of SCLE with previous PPI treatment may increase the risk of SCLE with other PPIs.
Fundic Gland Polyps: Long term PPI use, including rabeprazole, appears to be associated with an increased risk of fundic gland polyps. Most fundic gland polyps are asymptomatic. Patients with large or ulcerated polyps may be at risk of gastrointestinal bleeding or small intestinal blockage. Use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
Effects on ability to Drive and use Machines: Based on pharmacodynamic properties and the adverse events profile, it is unlikely that Rabeprazole sodium (PARIET) would cause an impairment of driving performance or compromise the ability to use machinery. If however, alertness is impaired due to somnolence, it is recommended that driving and operating complex machinery be avoided.
Use In Pregnancy & Lactation
Pregnancy: There are no data on the safety of rabeprazole in human pregnancy.
Reproduction studies performed in rats and rabbits have revealed no evidence of impaired fertility or harm to the fetus due to rabeprazole sodium, although low feto-placental transfer occurs in rats. Rabeprazole sodium (PARIET) is contraindicated during pregnancy.
Lactation: It is not known whether rabeprazole sodium is excreted in human breast milk. No studies in lactating women have been performed. Rabeprazole sodium is however excreted in rat mammary secretions. Therefore, Rabeprazole sodium (PARIET) should not be used during breast feeding.
Reproduction studies performed in rats and rabbits have revealed no evidence of impaired fertility or harm to the fetus due to rabeprazole sodium, although low feto-placental transfer occurs in rats. Rabeprazole sodium (PARIET) is contraindicated during pregnancy.
Lactation: It is not known whether rabeprazole sodium is excreted in human breast milk. No studies in lactating women have been performed. Rabeprazole sodium is however excreted in rat mammary secretions. Therefore, Rabeprazole sodium (PARIET) should not be used during breast feeding.
Adverse Reactions
Rabeprazole sodium (PARIET) tablets were generally well-tolerated during clinical trials. The observed undesirable effects have generally been mild/moderate and transient in nature and consistent between adults and adolescents. The most common adverse events are headache, diarrhea and nausea. Adverse reactions reported as more than isolated cases are listed as follows by system organ class and by frequency.
The following adverse reactions have been reported from clinical trial and post-marketing experience. However, of those adverse reactions reported in company sponsored clinical trials, only headache, diarrhea, abdominal pain, asthenia, flatulence, rash and dry mouth were associated with the use of Rabeprazole (PARIET) Tablets. Frequencies are defined as: common (>1/100, <1/10), uncommon (>1/1,000, <1/100), rare (>1/10,000, <1/1,000) and very rare (<1/10,000). (See table.)
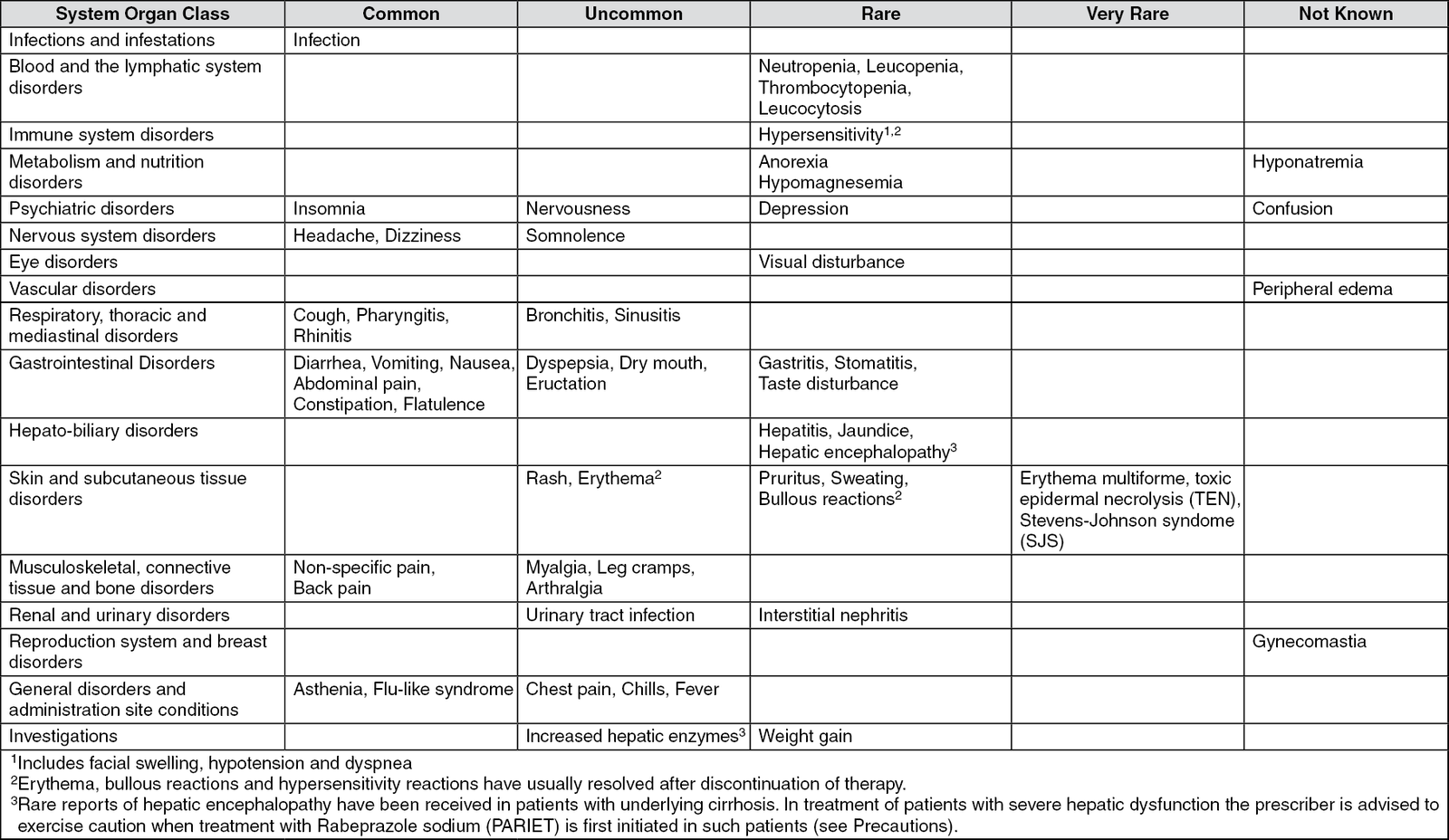
There have been post-marketing reports of bone fractures and post-marketing reports of subacute cutaneous lupus erythematosus (SCLE) (See Precautions).
The following adverse reactions have been reported from clinical trial and post-marketing experience. However, of those adverse reactions reported in company sponsored clinical trials, only headache, diarrhea, abdominal pain, asthenia, flatulence, rash and dry mouth were associated with the use of Rabeprazole (PARIET) Tablets. Frequencies are defined as: common (>1/100, <1/10), uncommon (>1/1,000, <1/100), rare (>1/10,000, <1/1,000) and very rare (<1/10,000). (See table.)

There have been post-marketing reports of bone fractures and post-marketing reports of subacute cutaneous lupus erythematosus (SCLE) (See Precautions).
Drug Interactions
Rabeprazole sodium, as is the case with other members of the proton pump inhibitor (PPI) class of compounds, is metabolized through the cytochrome P450 (CYP450) hepatic drug metabolizing system. Specifically, in vitro studies with human liver microsomes indicated that rabeprazole sodium is metabolized by isoenzymes CYP2C19 and CYP3A4.
Studies in healthy subjects have shown that rabeprazole sodium does not have pharmacokinetic or clinically significant interactions with warfarin, phenytoin, theophylline or diazepam (regardless of whether the subject was an extensive or poor diazepam metabolizer), each of which is metabolized by the CYP450 system.
Combination therapy with antimicrobials: 16 healthy volunteers were given 20 mg rabeprazole sodium, 1000 mg amoxicillin, 500 mg clarithromycin, or the combination of all 3 rabeprazole, amoxicillin, and clarithromycin (RAC) in a four way crossover study. The AUC and Cmax for clarithromycin and amoxicillin were similar during combined treatment compared to monotherapy. The rabeprazole AUC and Cmax increased by 11% and 34% and the 14-hydroxyclarithromycin (active metabolite of clarithromycin) AUC and Cmax increased by 42% and 46% during the combined treatment compared to values obtained during monotherapy. This increase in exposure to rabeprazole and the 14-hydroxyclarithromycin is not considered to be clinically significant.
Rabeprazole sodium produces a profound and long lasting inhibition of gastric acid secretion. An interaction with compounds whose absorption is pH dependent may occur. Co-administration of rabeprazole sodium with ketoconazole or itraconazole may result in a significant decrease in antifungal plasma levels. Therefore individual patients may need to be monitored to determine if a dosage adjustment is necessary when ketoconazole or itraconazole are taken concomitantly with Rabeprazole sodium (PARIET).
Co-administration of atazanavir 300 mg/ritonavir 100 mg with omeprazole or atazanavir 400 mg with lansoprazole to healthy volunteers resulted in a substantial reduction in atazanavir exposure. The absorption of atazanavir is pH dependent. Although co-administration with rabeprazole was not studied, similar results are expected with other proton pump inhibitors. Therefore, rabeprazole should not be co-administered with atazanavir.
In clinical trials, antacids were used concomitantly with the administration of Rabeprazole sodium (PARIET) and, in a specific study designed to define this interaction, no interaction with liquid antacids was observed.
Administration of rabeprazole sodium with a high fat meal may delay its absorption up to 4 hours or longer; however, the Cmax and the extent of absorption (AUC) are not altered.
Methotrexate: Case reports, published population pharmacokinetic studies, and retrospective analyses suggest that concomitant administration of FPIs and methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate. However, no formal drug interaction studies of methotrexate with PPIs have been conducted.
Studies in healthy subjects have shown that rabeprazole sodium does not have pharmacokinetic or clinically significant interactions with warfarin, phenytoin, theophylline or diazepam (regardless of whether the subject was an extensive or poor diazepam metabolizer), each of which is metabolized by the CYP450 system.
Combination therapy with antimicrobials: 16 healthy volunteers were given 20 mg rabeprazole sodium, 1000 mg amoxicillin, 500 mg clarithromycin, or the combination of all 3 rabeprazole, amoxicillin, and clarithromycin (RAC) in a four way crossover study. The AUC and Cmax for clarithromycin and amoxicillin were similar during combined treatment compared to monotherapy. The rabeprazole AUC and Cmax increased by 11% and 34% and the 14-hydroxyclarithromycin (active metabolite of clarithromycin) AUC and Cmax increased by 42% and 46% during the combined treatment compared to values obtained during monotherapy. This increase in exposure to rabeprazole and the 14-hydroxyclarithromycin is not considered to be clinically significant.
Rabeprazole sodium produces a profound and long lasting inhibition of gastric acid secretion. An interaction with compounds whose absorption is pH dependent may occur. Co-administration of rabeprazole sodium with ketoconazole or itraconazole may result in a significant decrease in antifungal plasma levels. Therefore individual patients may need to be monitored to determine if a dosage adjustment is necessary when ketoconazole or itraconazole are taken concomitantly with Rabeprazole sodium (PARIET).
Co-administration of atazanavir 300 mg/ritonavir 100 mg with omeprazole or atazanavir 400 mg with lansoprazole to healthy volunteers resulted in a substantial reduction in atazanavir exposure. The absorption of atazanavir is pH dependent. Although co-administration with rabeprazole was not studied, similar results are expected with other proton pump inhibitors. Therefore, rabeprazole should not be co-administered with atazanavir.
In clinical trials, antacids were used concomitantly with the administration of Rabeprazole sodium (PARIET) and, in a specific study designed to define this interaction, no interaction with liquid antacids was observed.
Administration of rabeprazole sodium with a high fat meal may delay its absorption up to 4 hours or longer; however, the Cmax and the extent of absorption (AUC) are not altered.
Methotrexate: Case reports, published population pharmacokinetic studies, and retrospective analyses suggest that concomitant administration of FPIs and methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate. However, no formal drug interaction studies of methotrexate with PPIs have been conducted.
Caution For Usage
Instructions for Use and Handling: No specific instructions needed.
Incompatibilities: Not applicable.
Incompatibilities: Not applicable.
Storage
Rabeprazole sodium (PARIET) should be stored at temperatures not exceeding 25°C and be protected from moisture after unsealing.
Shelf-Life: 36 months.
Shelf-Life: 36 months.
Action
Pharmacology: Pharmacodynamics: Mechanism of Action: Rabeprazole sodium belongs to the class of anti-secretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or H2-histamine antagonist properties, but suppress gastric acid secretion by the specific inhibition of the H+/K+-ATPase enzyme (the acid or proton pump). The effect is dose-related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus. Animal studies indicate that after administration, rabeprazole sodium rapidly disappears from both the plasma and gastric mucosa. As a weak base, rabeprazole is rapidly absorbed following all doses and is concentrated in the acid environment of the parietal cells. Rabeprazole is converted to the active sulphenamide form through protonation and it subsequently reacts with the available cysteines on the proton pump.
Anti-secretory Activity: After oral administration of a 20 mg dose of rabeprazole sodium, the onset of the anti-secretory effect occurs within one hour, with the maximum effect occurring within two to four hours. Inhibition of basal and food stimulated acid secretion 23 hours after the first dose of rabeprazole sodium are 69% and 82% respectively and the duration of inhibition lasts up to 48 hours. The inhibitory effect of rabeprazole sodium on acid secretion increases slightly with repeated once-daily dosing, achieving steady state inhibition after three days. When the drug is discontinued, secretory activity normalizes over 2 to 3 days.
Serum Gastrin Effects: In clinical studies, patients were treated once daily with 10 or 20 mg rabeprazole sodium, for up to 43 months duration. Serum gastrin levels increased during the first 2 to 8 weeks reflecting the inhibitory effects on acid secretion and remained stable while treatment was continued. Gastrin values returned to pre-treatment levels, usually within 1 to 2 weeks after discontinuation of therapy.
Human gastric biopsy specimens from the antrum and fundus from over 500 patients receiving rabeprazole or comparator treatment for up to 8 weeks have not detected changes in ECL cell history, degree of gastritis, incidence of atrophic gastritis, intestinal metaplasia or distribution of H. pylori infection. In over 250 patients followed for 36 months of continuous therapy, no significant change in findings present at baseline was observed.
Other Effects: Systemic effects of rabeprazole sodium in the CNS, cardiovascular and respiratory systems have not been found to date. Rabeprazole sodium, given in oral dose of 20 mg for 2 weeks, had no effect on thyroid function, carbohydrate metabolism or circulating levels of parathyroid hormone, cortisol, estrogen, testosterone, prolactin, cholecystokinin, secretin, glucagon, follicle-stimulating hormone (FSH), luteinizing hormone (LH), renin, aldosterone or somatotropic hormone.
Studies in healthy subjects have shown that rabeprazole sodium does not have clinically significant interactions with amoxicillin. Rabeprazole does not adversely influence plasma concentrations of amoxicillin or clarithromycin when co-administered for the purpose of eradicating upper gastrointestinal H. pylori infection.
Pharmacokinetics: Absorption: Rabeprazole sodium (PARIET) is an enteric-coated (gastro-resistant) tablet formulation of rabeprazole sodium. This presentation is necessary because rabeprazole is acid-labile. Absorption of rabeprazole therefore begins only after the tablet leaves the stomach. Absorption is rapid, with peak plasma levels of rabeprazole occurring approximately 3.5 hours after a 20 mg dose. Peak plasma concentrations (Cmax) of rabeprazole and AUC are linear over the dose range of 10 mg to 40 mg. Absolute bioavailability of an oral 20 mg dose (compared to intravenous administration) is about 52% due in large part of pre-systemic metabolism. Additionally the bioavailabilty does not appear to increase with repeat administration. In healthy subjects the plasma half-life is approximately one hour (range 0.7 to 1.5 hours), and the total body clearance is estimated to be 283±98 mL/min. There was no clinically relevant interaction with food. Neither food nor the time of day of administration of the treatment affect the absorption of rabeprazole sodium.
Distribution: Rabeprazole is approximately 97% bound to human plasma proteins.
Metabolism and excretion: Rabeprazole sodium, as is the case with other members of the proton pump inhibitor (PPI) class of compounds, is metabolized through the cytochrome P450 (CYP450) hepatic drug metabolizing system. In vitro studies with human liver microsomes indicated that rabeprazole sodium is metabolized by isoenzymes of CYP450 (CYP2C19 and CYP3A4). In these studies, at expected human plasma concentrations, rabeprazole neither induces nor inhibits CYP3A4; and although in vitro studies may not always be predictive of in vivo status these findings indicate that no interaction is expected between rabeprazole and cyclosporin. In humans, the thioether (M1) and carboxylic acid (M6) are the main plasma metabolites with the sulphone (M2), desmethyl-thioether (M4) and mercapturic acid conjugate (M5) minor metabolites observed at lower levels. Only the desmethyl metabolite (M3) has a small amount of anti-secretory activity but it is not present in plasma.
Following a single 20 mg 14C-labeled oral dose of rabeprazole sodium, no unchanged drug was excreted in the urine. Approximately 90% of the dose was eliminated in urine mainly as the two metabolites: A mercapturic acid conjugate (M5) and a carboxylic acid (M6), and 2 unknown metabolites. The remainder of the dose was recovered in feces.
Gender: Adjusted for body mass and height, there are no significant gender differences in pharmacokinetic parameters following a single 20 mg dose of rabeprazole.
Renal dysfunction: In patients with stable, end-stage, renal failure requiring maintenance hemodialysis (creatinine clearance ≤5 mL/min/1.73 m2), the disposition of rabeprazole was very similar to that in healthy volunteers. The AUC and the Cmax in these patients was about 35% lower than the corresponding parameters in healthy volunteers. The mean half-life of rabeprazole was 0.82 hour in healthy volunteers, 0.95 hour in patients during hemodialysis and 3.6 hours post dialysis. The clearance of the drug in patients with renal disease requiring maintenance hemodialysis was approximately twice that in healthy volunteers.
Hepatic dysfunction: Following a single 20 mg dose of rabeprazole to patients with chronic mild to moderate hepatic impairment the AUC doubled and there was a 2-3 fold increase in half-life of rabeprazole compared to the healthy volunteers. However, following a 20 mg dose daily for 7 days, the AUC had increased to only 1.5-fold and the Cmax to only 1.2-fold. The half-life of rabeprazole in patients with hepatic impairment was 12.3 hours compared to 2.1 hours in healthy volunteers. The pharmacodynamic response (gastric pH control) in the two groups was clinically comparable.
Elderly: Elimination of rabeprazole was somewhat decreased in the elderly. Following 7 days of daily dosing with 20 mg of rabeprazole sodium, the AUC approximately doubled, the Cmax increased by 60% and t1/2 increased by approximately 30% as compared to young healthy volunteers. However, there was no evidence of rabeprazole accumulation.
CYP2C19 Polymorphism: Following a 20 mg daily dose of rabeprazole for 7 days, CYP2C19 slow metabolizers, had AUC and t1/2 which were approximately 1.9 and 1.6 times the corresponding parameters in extensive metabolizers whilst Cmax had increased by only 40%.
Toxicology: Pre-clinical Safety Data: Pre-clinical effects were observed only at exposures sufficiently in excess of the maximum human exposure that make concerns for human safety negligible in respect of animal data.
Studies on mutagenicity gave equivocal results. Tests in mouse lymphoma cell line were positive, but in vivo micronucleus and in vivo and in vitro DNA repair tests were negative. Carcinogenicity studies revealed no special hazard for humans.
Anti-secretory Activity: After oral administration of a 20 mg dose of rabeprazole sodium, the onset of the anti-secretory effect occurs within one hour, with the maximum effect occurring within two to four hours. Inhibition of basal and food stimulated acid secretion 23 hours after the first dose of rabeprazole sodium are 69% and 82% respectively and the duration of inhibition lasts up to 48 hours. The inhibitory effect of rabeprazole sodium on acid secretion increases slightly with repeated once-daily dosing, achieving steady state inhibition after three days. When the drug is discontinued, secretory activity normalizes over 2 to 3 days.
Serum Gastrin Effects: In clinical studies, patients were treated once daily with 10 or 20 mg rabeprazole sodium, for up to 43 months duration. Serum gastrin levels increased during the first 2 to 8 weeks reflecting the inhibitory effects on acid secretion and remained stable while treatment was continued. Gastrin values returned to pre-treatment levels, usually within 1 to 2 weeks after discontinuation of therapy.
Human gastric biopsy specimens from the antrum and fundus from over 500 patients receiving rabeprazole or comparator treatment for up to 8 weeks have not detected changes in ECL cell history, degree of gastritis, incidence of atrophic gastritis, intestinal metaplasia or distribution of H. pylori infection. In over 250 patients followed for 36 months of continuous therapy, no significant change in findings present at baseline was observed.
Other Effects: Systemic effects of rabeprazole sodium in the CNS, cardiovascular and respiratory systems have not been found to date. Rabeprazole sodium, given in oral dose of 20 mg for 2 weeks, had no effect on thyroid function, carbohydrate metabolism or circulating levels of parathyroid hormone, cortisol, estrogen, testosterone, prolactin, cholecystokinin, secretin, glucagon, follicle-stimulating hormone (FSH), luteinizing hormone (LH), renin, aldosterone or somatotropic hormone.
Studies in healthy subjects have shown that rabeprazole sodium does not have clinically significant interactions with amoxicillin. Rabeprazole does not adversely influence plasma concentrations of amoxicillin or clarithromycin when co-administered for the purpose of eradicating upper gastrointestinal H. pylori infection.
Pharmacokinetics: Absorption: Rabeprazole sodium (PARIET) is an enteric-coated (gastro-resistant) tablet formulation of rabeprazole sodium. This presentation is necessary because rabeprazole is acid-labile. Absorption of rabeprazole therefore begins only after the tablet leaves the stomach. Absorption is rapid, with peak plasma levels of rabeprazole occurring approximately 3.5 hours after a 20 mg dose. Peak plasma concentrations (Cmax) of rabeprazole and AUC are linear over the dose range of 10 mg to 40 mg. Absolute bioavailability of an oral 20 mg dose (compared to intravenous administration) is about 52% due in large part of pre-systemic metabolism. Additionally the bioavailabilty does not appear to increase with repeat administration. In healthy subjects the plasma half-life is approximately one hour (range 0.7 to 1.5 hours), and the total body clearance is estimated to be 283±98 mL/min. There was no clinically relevant interaction with food. Neither food nor the time of day of administration of the treatment affect the absorption of rabeprazole sodium.
Distribution: Rabeprazole is approximately 97% bound to human plasma proteins.
Metabolism and excretion: Rabeprazole sodium, as is the case with other members of the proton pump inhibitor (PPI) class of compounds, is metabolized through the cytochrome P450 (CYP450) hepatic drug metabolizing system. In vitro studies with human liver microsomes indicated that rabeprazole sodium is metabolized by isoenzymes of CYP450 (CYP2C19 and CYP3A4). In these studies, at expected human plasma concentrations, rabeprazole neither induces nor inhibits CYP3A4; and although in vitro studies may not always be predictive of in vivo status these findings indicate that no interaction is expected between rabeprazole and cyclosporin. In humans, the thioether (M1) and carboxylic acid (M6) are the main plasma metabolites with the sulphone (M2), desmethyl-thioether (M4) and mercapturic acid conjugate (M5) minor metabolites observed at lower levels. Only the desmethyl metabolite (M3) has a small amount of anti-secretory activity but it is not present in plasma.
Following a single 20 mg 14C-labeled oral dose of rabeprazole sodium, no unchanged drug was excreted in the urine. Approximately 90% of the dose was eliminated in urine mainly as the two metabolites: A mercapturic acid conjugate (M5) and a carboxylic acid (M6), and 2 unknown metabolites. The remainder of the dose was recovered in feces.
Gender: Adjusted for body mass and height, there are no significant gender differences in pharmacokinetic parameters following a single 20 mg dose of rabeprazole.
Renal dysfunction: In patients with stable, end-stage, renal failure requiring maintenance hemodialysis (creatinine clearance ≤5 mL/min/1.73 m2), the disposition of rabeprazole was very similar to that in healthy volunteers. The AUC and the Cmax in these patients was about 35% lower than the corresponding parameters in healthy volunteers. The mean half-life of rabeprazole was 0.82 hour in healthy volunteers, 0.95 hour in patients during hemodialysis and 3.6 hours post dialysis. The clearance of the drug in patients with renal disease requiring maintenance hemodialysis was approximately twice that in healthy volunteers.
Hepatic dysfunction: Following a single 20 mg dose of rabeprazole to patients with chronic mild to moderate hepatic impairment the AUC doubled and there was a 2-3 fold increase in half-life of rabeprazole compared to the healthy volunteers. However, following a 20 mg dose daily for 7 days, the AUC had increased to only 1.5-fold and the Cmax to only 1.2-fold. The half-life of rabeprazole in patients with hepatic impairment was 12.3 hours compared to 2.1 hours in healthy volunteers. The pharmacodynamic response (gastric pH control) in the two groups was clinically comparable.
Elderly: Elimination of rabeprazole was somewhat decreased in the elderly. Following 7 days of daily dosing with 20 mg of rabeprazole sodium, the AUC approximately doubled, the Cmax increased by 60% and t1/2 increased by approximately 30% as compared to young healthy volunteers. However, there was no evidence of rabeprazole accumulation.
CYP2C19 Polymorphism: Following a 20 mg daily dose of rabeprazole for 7 days, CYP2C19 slow metabolizers, had AUC and t1/2 which were approximately 1.9 and 1.6 times the corresponding parameters in extensive metabolizers whilst Cmax had increased by only 40%.
Toxicology: Pre-clinical Safety Data: Pre-clinical effects were observed only at exposures sufficiently in excess of the maximum human exposure that make concerns for human safety negligible in respect of animal data.
Studies on mutagenicity gave equivocal results. Tests in mouse lymphoma cell line were positive, but in vivo micronucleus and in vivo and in vitro DNA repair tests were negative. Carcinogenicity studies revealed no special hazard for humans.
MedsGo Class
Features
Dosage
10 mg
Ingredients
- Rabeprazole
Packaging
Tablet 1's
Generic Name
Rabeprazole Sodium
Registration Number
DR-XY25304
Classification
Prescription Drug (RX)
Product Questions
Questions

