Indications/Uses
20 mg capsule: For the short-term treatment and symptomatic relief of heartburn that occurs two or more days per week (frequent heartburn).
40 mg capsule: For the short-term and maintenance treatment of the following conditions: Duodenal ulcer; Gastric ulcer; Pathological hypersecretory conditions (e.g., Zollinger-Ellison Syndrome, multiple endocrine adenomas, and systemic mastocytis); Gastroesophageal Reflux Disease (GERD) and other symptoms associated with the disease; NSAID-associated gastric and duodenal ulcers or erosions; Acid-related dyspepsia; Erosive Esophagitis; Helicobacter pylori eradication regimens in peptic ulcer disease.
Prophylaxis of acid aspiration during general anesthesia.
Powder for injection: For the short term treatment of the following conditions when oral medication is not feasible: Duodenal ulcer; Gastric ulcer; Ulcerative esophagitis o Zollinger-Ellison Syndrome; Gastroesophageal Reflux Disease (GERD); NSAID-induced gastrointestinal complications (e.g., perioperative therapy).
Prophylaxis of acid reflux.
40 mg capsule: For the short-term and maintenance treatment of the following conditions: Duodenal ulcer; Gastric ulcer; Pathological hypersecretory conditions (e.g., Zollinger-Ellison Syndrome, multiple endocrine adenomas, and systemic mastocytis); Gastroesophageal Reflux Disease (GERD) and other symptoms associated with the disease; NSAID-associated gastric and duodenal ulcers or erosions; Acid-related dyspepsia; Erosive Esophagitis; Helicobacter pylori eradication regimens in peptic ulcer disease.
Prophylaxis of acid aspiration during general anesthesia.
Powder for injection: For the short term treatment of the following conditions when oral medication is not feasible: Duodenal ulcer; Gastric ulcer; Ulcerative esophagitis o Zollinger-Ellison Syndrome; Gastroesophageal Reflux Disease (GERD); NSAID-induced gastrointestinal complications (e.g., perioperative therapy).
Prophylaxis of acid reflux.
Dosage/Direction for Use
20 mg capsule: Adult dose: Orally (by mouth), 1 capsule once a day 30 minutes to 1 hour before breakfast with a full glass of water.
Take the medicine for 14 days.
Or, as directed by a doctor.
Do not take more than 20 mg (1 capsule) per day.
Do not take a 14-day course more often than every 4 months unless directed by a doctor.
Capsules should be swallowed whole. Do not split, crush or chew.
For children under 18 years old, ask a doctor.
Alternative Administration Option: For patients who are unable to swallow the capsule whole, the capsule may be opened and the contents swallowed directly with a half glass of liquid or after mixing with a slightly acidic fluid such as applesauce, fruit juice and yoghurt or in non-carbonated water. The mixture should be taken immediately and should not be stored for future use. The pellets must not be chewed or crushed.
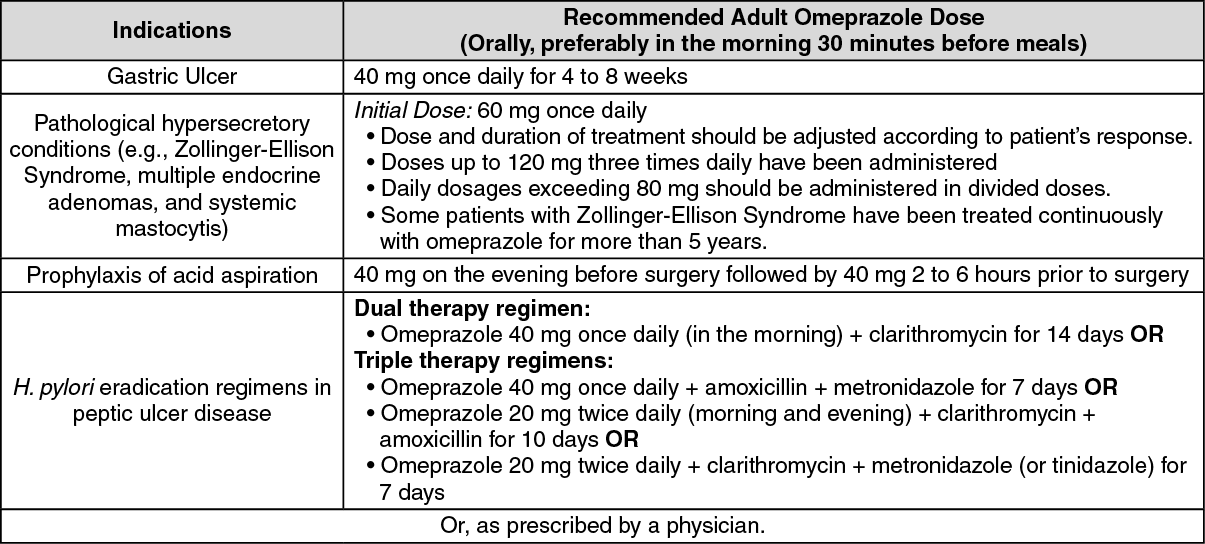
40 mg capsule: Capsules should be swallowed whole. Do not split, crush or chew. (See table.)
Take the medicine for 14 days.
Or, as directed by a doctor.
Do not take more than 20 mg (1 capsule) per day.
Do not take a 14-day course more often than every 4 months unless directed by a doctor.
Capsules should be swallowed whole. Do not split, crush or chew.
For children under 18 years old, ask a doctor.
Alternative Administration Option: For patients who are unable to swallow the capsule whole, the capsule may be opened and the contents swallowed directly with a half glass of liquid or after mixing with a slightly acidic fluid such as applesauce, fruit juice and yoghurt or in non-carbonated water. The mixture should be taken immediately and should not be stored for future use. The pellets must not be chewed or crushed.
40 mg capsule: Capsules should be swallowed whole. Do not split, crush or chew. (See table.)
Powder for injection: Omeprazole (Omepron) should be administered only by slow intravenous (IV) injection (not less than 2.5 minutes) at a rate of no greater than 4 mL/minute.
Omeprazole (Omepron) IV injection is NOT intended for use as IV infusion.
Recommended Dose: 40 mg once daily.
Patients with Zollinger-Ellison Syndrome may require a higher dose and/or several doses. Individual dosage adjustment is necessary in these patients.
Parenteral omeprazole should be shifted to oral omeprazole as soon as feasible. Usual treatment period for intravenous omeprazole is 2-3 days.
Inspect the solution for particulate matter and discoloration before administration.
If intravenous therapy is necessary for more than three days, dosage must be adjusted and reduced to 10-20 mg per day based on patient's response.
Preparation of Dosage Form: Reconstitute powder with 10 mL of the solvent provided. No other solution should be used.
Stability: Omeprazole should be used within 4 hours after reconstitution.
Omeprazole (Omepron) IV injection is NOT intended for use as IV infusion.
Recommended Dose: 40 mg once daily.
Patients with Zollinger-Ellison Syndrome may require a higher dose and/or several doses. Individual dosage adjustment is necessary in these patients.
Parenteral omeprazole should be shifted to oral omeprazole as soon as feasible. Usual treatment period for intravenous omeprazole is 2-3 days.
Inspect the solution for particulate matter and discoloration before administration.
If intravenous therapy is necessary for more than three days, dosage must be adjusted and reduced to 10-20 mg per day based on patient's response.
Preparation of Dosage Form: Reconstitute powder with 10 mL of the solvent provided. No other solution should be used.
Stability: Omeprazole should be used within 4 hours after reconstitution.
Overdosage
Two cases of omeprazole overdose were characterized by drowsiness, headache (possibly due to a metabolite) and tachycardia. Both patients recovered without specific treatment.
Intravenous doses of 270 mg given in a day and up to 650 mg over a three-day period in clinical trials did not show any dose-related adverse reactions. As in all cases where overdosing is suspected, treatment should be symptomatic and supportive.
Intravenous doses of 270 mg given in a day and up to 650 mg over a three-day period in clinical trials did not show any dose-related adverse reactions. As in all cases where overdosing is suspected, treatment should be symptomatic and supportive.
Administration
Should be taken on an empty stomach: Take 30 min-1 hr before breakfast. Swallow whole, do not split/crush/chew. For patients w/ swallowing difficulty, cap may be opened & pellets mixed w/ a slightly acidic fluid (e.g. applesauce, fruit juice, yoghurt, non-carbonated water). Take mixt immediately & avoid chewing/crushing pellets. Do not store mixt for future use.
Special Precautions
20 mg capsule: Had heartburn for more than three months.
Heartburn with lightheadedness, sweating or dizziness.
Chest pain or shoulder pain with lightheadedness, shortness of breath, sweating, or pain spreading to the arms, neck, or shoulders.
Frequent chest pain.
Frequent wheezing related to heartburn.
40 mg capsule and Powder for injection: Gastric malignancy should be excluded at initiation of treatment and at follow-up of gastric ulcer therapy.
Atrophic gastritis has been reported occasionally in gastric corpus biopsies from patients on long-term omeprazole therapy.
The use of acid-suppressants such as omeprazole may increase a patient's risk of community-acquired pneumonia.
Heartburn with lightheadedness, sweating or dizziness.
Chest pain or shoulder pain with lightheadedness, shortness of breath, sweating, or pain spreading to the arms, neck, or shoulders.
Frequent chest pain.
Frequent wheezing related to heartburn.
40 mg capsule and Powder for injection: Gastric malignancy should be excluded at initiation of treatment and at follow-up of gastric ulcer therapy.
Atrophic gastritis has been reported occasionally in gastric corpus biopsies from patients on long-term omeprazole therapy.
The use of acid-suppressants such as omeprazole may increase a patient's risk of community-acquired pneumonia.
Adverse Reactions
Omeprazole is generally well-tolerated and most adverse reactions have been mild and transient.
Nervous System Effects: Headache, vertigo, dizziness, weakness (asthenia), pain, fatigue, malaise, paresthesia, hemifacial dysesthesia, and psychic disturbances (e.g., depression, aggression, confusion, anxiety, agitation, insomnia, nervousness, tremors, apathy, dream abnormalities, somnolence, and hallucinations) have been reported but not directly attributable to the drug in many cases.
Gastrointestinal Effects: Constipation and diarrhea, nausea, vomiting, abdominal pain, flatulence, and acid regurgitation. Occasionally, dysphagia, abdominal swelling, irritable colon, fecal discoloration, pancreatitis (sometimes fatal), esophageal candidiasis, gastric hypermotility, mucosal atrophy of the tongue, taste disturbances, anorexia, stomatitis, dry mouth, dysgeusia, and tongue discoloration have been reported. Benign gastric fundic polyps have been rarely reported but resolve upon discontinuation of omeprazole therapy.
Musculoskeletal: Back pain, muscle cramps, myalgia, muscle weakness, arthralgia, joint pain, and leg pain have been occasionally reported with the use of omeprazole.
Observational studies have shown that long term use and high dose proton pump inhibitor therapy may increase the risk of hip fracture in patients 50 years and older.
Dermatologic and Sensitivity Reactions: Rash, severe generalized reactions (e.g., toxic epidermal necrolysis), Stevens-Johnson Syndrome, erythema multiforme, exfoliative dermatitis, and lichenoid eruptions. Other effects include skin inflammation, photosensitivity, urticaria, purpura and/or petechiae, bullous eruption, angioedema, pruritus, alopecia, dry skin, and hyperhydrosis. Allergic reactions, including rare cases of anaphylaxis have also been reported.
Genitourinary Effects: Acute interstitial nephritis and sexual disturbances such as priaprism have been occasionally reported with the use of omeprazole. Urinary tract infection, microscopic pyuria, urinary frequency, elevated serum creatinine concentration, proteinuria, hematuria, glycosuria, and testicular pain have also been reported but in many cases not attributed to omeprazole.
Hepatic Effects: There have been rare reports of increases in serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), γ-glutamyltranspeptidase (GGTP), alkaline phosphatase and bilirubin concentrations. Symptomatic liver disease including hepatocellular, cholestatic, or mixed hepatitis, jaundice, liver necrosis, hepatic failure, and hepatic encephalopathy have occurred rarely.
Respiratory Effects: Upper respiratory tract infections and cough. Epistaxis and pharyngeal pain have been reported occasionally. Acute respiratory distress syndrome, respiratory failure, and pneumothorax have occurred in some patients. Other effects that were reported with combined omeprazole and clarithromycin therapy were rhinitis, pharyngitis and flu syndrome.
The use of proton pump inhibitors has been associated with an increased risk of developing community-acquired pneumonia. These drugs should only be used when clearly needed using the lowest effective dose in patients with severe community-acquired pneumonia.
Cardiovascular Effects: Chest pain, angina pectoris, tachycardia, bradycardia, palpitation, hypotension, hypertension, and peripheral edema have been reported occasionally. Atrial fibrillation, ventricular tachycardia, bradycardia, palpitation, and peripheral edema have also been reported.
Hematologic Effects: Leukocytosis, neutropenia, pancytopenia, agranulocytosis, leukopenia, anemia, hemolytic anemia, and thrombocytopenia have been reported rarely.
Ocular Effects: Blurred vision, ocular irritation, dry eye syndrome, optic atrophy, anterior ischemic optic neuropathy, optic neuritis, and double vision have been reported.
Other Adverse Effects: Fever, tinnitus, weight gain, acute gout, hypophosphatemia, hypocalcemia, fluid overload, hyperglycemia, hypomagnesemia, hypokalemia, sweating, hyponatremia, and otitis media. Edema, gynecomastia, oral candidiasis, and candidal infection have also occurred.
Nervous System Effects: Headache, vertigo, dizziness, weakness (asthenia), pain, fatigue, malaise, paresthesia, hemifacial dysesthesia, and psychic disturbances (e.g., depression, aggression, confusion, anxiety, agitation, insomnia, nervousness, tremors, apathy, dream abnormalities, somnolence, and hallucinations) have been reported but not directly attributable to the drug in many cases.
Gastrointestinal Effects: Constipation and diarrhea, nausea, vomiting, abdominal pain, flatulence, and acid regurgitation. Occasionally, dysphagia, abdominal swelling, irritable colon, fecal discoloration, pancreatitis (sometimes fatal), esophageal candidiasis, gastric hypermotility, mucosal atrophy of the tongue, taste disturbances, anorexia, stomatitis, dry mouth, dysgeusia, and tongue discoloration have been reported. Benign gastric fundic polyps have been rarely reported but resolve upon discontinuation of omeprazole therapy.
Musculoskeletal: Back pain, muscle cramps, myalgia, muscle weakness, arthralgia, joint pain, and leg pain have been occasionally reported with the use of omeprazole.
Observational studies have shown that long term use and high dose proton pump inhibitor therapy may increase the risk of hip fracture in patients 50 years and older.
Dermatologic and Sensitivity Reactions: Rash, severe generalized reactions (e.g., toxic epidermal necrolysis), Stevens-Johnson Syndrome, erythema multiforme, exfoliative dermatitis, and lichenoid eruptions. Other effects include skin inflammation, photosensitivity, urticaria, purpura and/or petechiae, bullous eruption, angioedema, pruritus, alopecia, dry skin, and hyperhydrosis. Allergic reactions, including rare cases of anaphylaxis have also been reported.
Genitourinary Effects: Acute interstitial nephritis and sexual disturbances such as priaprism have been occasionally reported with the use of omeprazole. Urinary tract infection, microscopic pyuria, urinary frequency, elevated serum creatinine concentration, proteinuria, hematuria, glycosuria, and testicular pain have also been reported but in many cases not attributed to omeprazole.
Hepatic Effects: There have been rare reports of increases in serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), γ-glutamyltranspeptidase (GGTP), alkaline phosphatase and bilirubin concentrations. Symptomatic liver disease including hepatocellular, cholestatic, or mixed hepatitis, jaundice, liver necrosis, hepatic failure, and hepatic encephalopathy have occurred rarely.
Respiratory Effects: Upper respiratory tract infections and cough. Epistaxis and pharyngeal pain have been reported occasionally. Acute respiratory distress syndrome, respiratory failure, and pneumothorax have occurred in some patients. Other effects that were reported with combined omeprazole and clarithromycin therapy were rhinitis, pharyngitis and flu syndrome.
The use of proton pump inhibitors has been associated with an increased risk of developing community-acquired pneumonia. These drugs should only be used when clearly needed using the lowest effective dose in patients with severe community-acquired pneumonia.
Cardiovascular Effects: Chest pain, angina pectoris, tachycardia, bradycardia, palpitation, hypotension, hypertension, and peripheral edema have been reported occasionally. Atrial fibrillation, ventricular tachycardia, bradycardia, palpitation, and peripheral edema have also been reported.
Hematologic Effects: Leukocytosis, neutropenia, pancytopenia, agranulocytosis, leukopenia, anemia, hemolytic anemia, and thrombocytopenia have been reported rarely.
Ocular Effects: Blurred vision, ocular irritation, dry eye syndrome, optic atrophy, anterior ischemic optic neuropathy, optic neuritis, and double vision have been reported.
Other Adverse Effects: Fever, tinnitus, weight gain, acute gout, hypophosphatemia, hypocalcemia, fluid overload, hyperglycemia, hypomagnesemia, hypokalemia, sweating, hyponatremia, and otitis media. Edema, gynecomastia, oral candidiasis, and candidal infection have also occurred.
Action
Proton Pump Inhibitor.
Pharmacology: Omeprazole is a proton pump inhibitor (PPI) that belongs to the substituted benzimidazoles class of antisecretory compounds. It suppresses gastric acid secretion by specific inhibition of the H+/K+ ATPase enzyme system at the secretory surface of the gastric parietal cell. This enzyme system is regarded as the acid (proton) pump within the gastric mucosa hence, omeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated acid secretion.
Pharmacokinetics: Bioavailability: Omeprazole's apparent volume of distribution is approximately 0.3 L/kg in healthy subjects and in patients with renal insufficiency. The volume of distribution is slightly decreased in elderly patients and in patients with hepatic insufficiency. Omeprazole is highly protein bound with about 95% bound to plasma proteins.
Omeprazole is completely metabolized mainly in the liver via the cytochrome P450 system (CYP). A major part of its metabolism is dependent on CYP2C19 (S-mephenytoin hydroxylase) which is responsible for the formation of hydroxyomeprazole, its major metabolite in the plasma. The average half-life after IV administration is approximately 40 minutes and total plasma clearance is 0.3 to 0.6 L/min.
About 80% of the metabolites are excreted in the urine and the remaining metabolites are seen in the feces. Patients with impaired liver function have increased elimination half-life; however, omeprazole has not shown any accumulation with once daily oral dosing.
Pharmacology: Omeprazole is a proton pump inhibitor (PPI) that belongs to the substituted benzimidazoles class of antisecretory compounds. It suppresses gastric acid secretion by specific inhibition of the H+/K+ ATPase enzyme system at the secretory surface of the gastric parietal cell. This enzyme system is regarded as the acid (proton) pump within the gastric mucosa hence, omeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated acid secretion.
Pharmacokinetics: Bioavailability: Omeprazole's apparent volume of distribution is approximately 0.3 L/kg in healthy subjects and in patients with renal insufficiency. The volume of distribution is slightly decreased in elderly patients and in patients with hepatic insufficiency. Omeprazole is highly protein bound with about 95% bound to plasma proteins.
Omeprazole is completely metabolized mainly in the liver via the cytochrome P450 system (CYP). A major part of its metabolism is dependent on CYP2C19 (S-mephenytoin hydroxylase) which is responsible for the formation of hydroxyomeprazole, its major metabolite in the plasma. The average half-life after IV administration is approximately 40 minutes and total plasma clearance is 0.3 to 0.6 L/min.
About 80% of the metabolites are excreted in the urine and the remaining metabolites are seen in the feces. Patients with impaired liver function have increased elimination half-life; however, omeprazole has not shown any accumulation with once daily oral dosing.
MedsGo Class
Antacids, Antireflux Agents & Antiulcerants
Features
Dosage
40 mg
Ingredients
- Omeprazole
Packaging
Delayed-Release Capsule 28's
Generic Name
Omeprazole
Registration Number
DR-XY40006
Classification
Prescription Drug (RX)
Product Questions
Questions

