Indications/Uses
Treatment of gastric mucosal lesions (erosions, bleeding, redness and edema) in the following conditions: Acute gastritis and acute exacerbation of chronic gastritis. Gastric ulcers.
Dosage/Direction for Use
Adult: Gastric Mucosal Lesions (Erosion, Bleeding, Redness and Edema) in Acute Gastritis and Acute Exacerbation of Chronic Gastritis: Usual Dose: 1 tab orally 3 times daily.
Gastric Ulcers: Usual Dose: 1 tab orally 3 times daily (in the morning, in the evening and before bedtime).
Gastric Ulcers: Usual Dose: 1 tab orally 3 times daily (in the morning, in the evening and before bedtime).
Administration
May be taken with or without food.
Contraindications
Patients with a history of hypersensitivity to any ingredient of this drug.
Special Precautions
Use in Pregnancy & Lactation: Mucosta should be used by pregnant or possibly pregnant women only if the anticipated therapeutic benefit is thought to outweigh any potential risk. (The safety of rebamipide in pregnant women has not been established.)
Nursing should be interrupted when Mucosta is administered to a nursing woman. (Rat studies showed that rebamipide is distributed in the breast milk.)
Use in Children: The safety of Mucosta in children has not been established (clinical experience in children is insufficient).
Use in Elderly: Special care should be given to elderly patients to minimize the risk of GI disorders because these patients may be physiologically more sensitive to Mucosta than younger patients.
Nursing should be interrupted when Mucosta is administered to a nursing woman. (Rat studies showed that rebamipide is distributed in the breast milk.)
Use in Children: The safety of Mucosta in children has not been established (clinical experience in children is insufficient).
Use in Elderly: Special care should be given to elderly patients to minimize the risk of GI disorders because these patients may be physiologically more sensitive to Mucosta than younger patients.
Use In Pregnancy & Lactation
Mucosta should be used by pregnant or possibly pregnant women only if the anticipated therapeutic benefit is thought to outweigh any potential risk. (The safety of rebamipide in pregnant women has not been established.)
Nursing should be interrupted when Mucosta is administered to a nursing woman. (Rat studies showed that rebamipide is distributed in the breast milk.)
Nursing should be interrupted when Mucosta is administered to a nursing woman. (Rat studies showed that rebamipide is distributed in the breast milk.)
Adverse Reactions
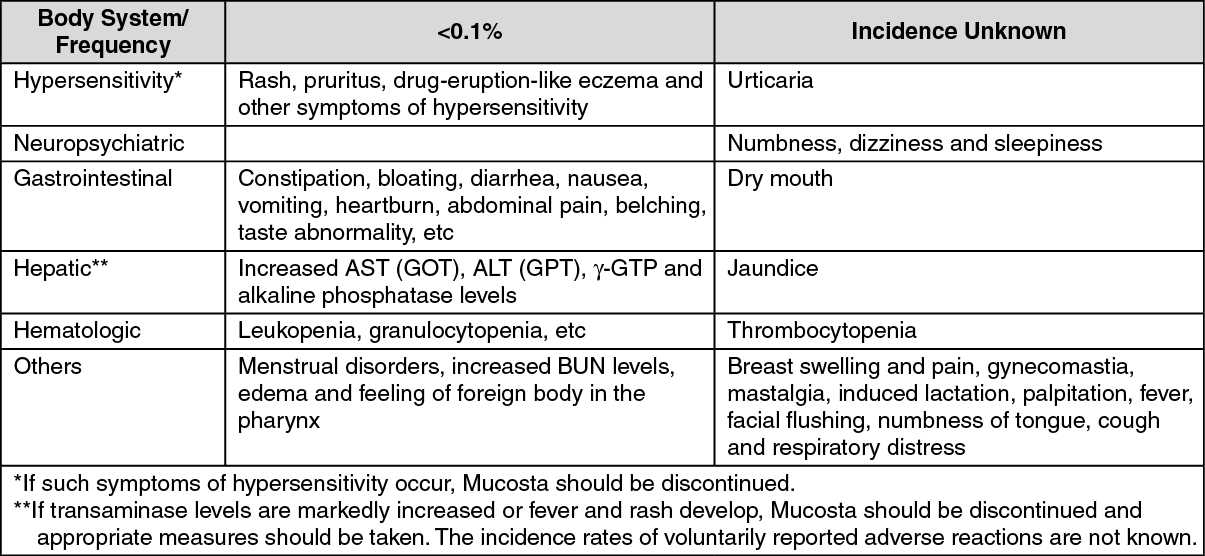
Of 10,407 patients treated, adverse reactions including abnormal laboratory findings, were reported in 54 patients (0.54%). Of 3035 patients >65 years, adverse reactions were noted in 18 patients (0.59%). The nature and incidence of adverse reactions were not different between the elderly and younger patients. The summary of data as follows includes adverse reactions voluntarily reported after marketing (figures are total cases reported from the time of approval up to June 2001).
Hypersensitivity: Rash, pruritus and eczematous drug eruption may rarely occur. If such signs of hypersensitivity reactions develop, Mucosta should be discontinued.
Gastrointestinal: Dry mouth, constipation, sensation of abdominal enlargement, diarrhea, nausea, vomiting, heartburn, abdominal pain and belching may rarely occur.
Hepatic: Signs of hepatic function disorder including increased GOT, GPT, γ-GTP and alkaline phosphatase levels may rarely occur.
Hematologic: Leukopenia may rarely occur.
Others: Mammary gland expansion, nonpuerperal lactation, menstrual disorder, dizziness, increase in BUN level, edema and sensation of foreign body in the pharynx may rarely occur. (See table.)
Hypersensitivity: Rash, pruritus and eczematous drug eruption may rarely occur. If such signs of hypersensitivity reactions develop, Mucosta should be discontinued.
Gastrointestinal: Dry mouth, constipation, sensation of abdominal enlargement, diarrhea, nausea, vomiting, heartburn, abdominal pain and belching may rarely occur.
Hepatic: Signs of hepatic function disorder including increased GOT, GPT, γ-GTP and alkaline phosphatase levels may rarely occur.
Hematologic: Leukopenia may rarely occur.
Others: Mammary gland expansion, nonpuerperal lactation, menstrual disorder, dizziness, increase in BUN level, edema and sensation of foreign body in the pharynx may rarely occur. (See table.)
Storage
Store at a temperature not exceeding 25°C.
Shelf-Life: 24 months.
Shelf-Life: 24 months.
Action
Cytoprotective agent.
Pharmacology: Experiments Using Animal Models: Preventive or Healing Effects in Gastric Ulcer Models: Rebamipide inhibited gastric mucosal damage in various experimental rat models of ulcers, including ulcers induced by water-immersion restraint stress, aspirin, indomethacin, histamine, serotonin and pyloric ligation. It also protected the mucosa from damage caused by other ulcerogenic agents that, presumably yield oxygen-free radicals, including mucosal ischemia and reperfusion, administration of platelet activating factor (PAF) or diethyldithiocarbamate (DDC) and administration of indomethacin under stress conditions. The drug promoted healing of gastric ulcers and suppressed recurrence or relapse of ulcers even at 120-140 days after ulcer production in rat acetic acid-induced ulcer model.
Preventive or Healing Effects in Gastritis Models: Rebamipide inhibited the occurrence of taurocholic acid-induced gastritis and promoted healing of mucosal inflammation in a rat gastritis model.
Mechanism of Action: Prostaglandin Increasing Effect: Rebamipide increased the endogenous prostaglandin E2 (PGE2) content in the gastric mucosa in rats. The drug also increased PGE2 and PGI2 content in the gastric juice, as well as the content of 15-keto-13, 14-PGE2, a metabolite of PGE2. In healthy male subjects, the drug again revealed the effect of increasing the PGE2 content in the gastric mucosa and protected the gastric mucosa from injury caused by ethanol loading.
Cytoprotective Effect: Rebamipide exhibited a gastric cytoprotective effect by inhibiting mucosal damage induced by ethanol, strong acid and strong base in rat experiments. The drug also protected gastric epithelial cells in vitro against aspirin- or taurocholic acid-induced injury in cultured cells obtained from rabbit fetuses.
In healthy male subjects, the drug inhibited gastric mucosal injury induced by aspirin, ethanol and HCl-ethanol loading.
Mucus-Increasing Effect: Rebamipide promoted enzyme activity to synthesize high molecular glycoproteins and increased the amounts of gastric surface mucus and soluble mucus in rats. Endogenous PGs are not involved in the increase in soluble mucus.
Mucosal Blood Flow-Increasing Effect: Rebamipide increased gastric mucosal blood flow and improved hemodynamics after blood loss in rats.
Activity on Mucosal Barrier: Rebamipide did not ordinarily affect the gastric transmucosal potential difference in rats, but did inhibit a decrease in the potential difference of ethanol in the stomach of rats.
Activity on Gastric Alkaline Secretion: Rebamipide promotes alkaline secretion in the stomach of rats.
Activity on Mucosal Cell Turnover: Rebamipide activated gastric mucosal cell proliferation and increased the number of epithelial cells in the stomach of rats.
Effect on Gastric Mucous Repair: Rebamipide restored the bile acid- or hydrogen peroxide-induced retardation of artificial wound-repair in cultured rabbit gastric epithelial cells.
Effect on Gastric Secretion: Rebamipide did not alter either basal secretion of gastric juice or stimulatory acid secretion.
Effects on Oxygen-Free Radicals: Rebamipide scavenged hydroxyl radicals and suppressed superoxide production by polymorphonuclear leukocytes. The drug also protected the gastric mucosa from damage by oxygen-free radicals released from neutrophils stimulated by Helicobacter pylori in vitro. The drug inhibited mucosal damage and reduced the content of lipid peroxide in the gastric mucosa of rats treated with indomethacin under stressed conditions.
Effects on Inflammatory Cellular Infiltration in the Gastric Mucosa: Rebamipide prevented inflammatory cellular infiltration in rat models of taurocholic acid-induced gastritis, NSAID-induced gastric mucosal damage and ischemia-perfusion-induced gastric mucosal damage.
Effects on Inflammatory Cytokine Release (Interleukin-8) in the Gastric Mucosa: Rebamipide suppressed the increased production and release of interleukin-8 from human gastric mucosa in the presence of Helicobacter pylori in vitro. The drug also inhibited the activation of NF-KB and suppressed the expression of interleukin-8 mRNA in epithelial cells in vitro. Clinical Studies: Clinical Efficacy in Gastric Ulcer: Mucosta was studied in patients with gastric ulcer using endoscopy for objective drug evaluation. In the final endoscopic assessment, the drug achieved complete healing rate in 60% (200/335) of the patients studied and near-complete healing in 67% (224/335). Clinical usefulness of this drug based on efficacy and safety were demonstrated in a double-blind study. A 6-month follow-up of 67 patients who showed healing at a daily dose of 300 mg revealed that recurrence occurred in only 4 patients (Yulex6%).
Clinical Efficacy in Acute Gastritis and Acute Exacerbation of Chronic Gastritis: Mucosta was studied in patients with acute gastritis or acute exacerbation of chronic gastritis. The drug achieved an 80% (370/461) global efficacy rate in patients evaluated with 76% (351/461) showing moderate or marked improvement. The drug's clinical usefulness can be reproduced in a double-blind study.
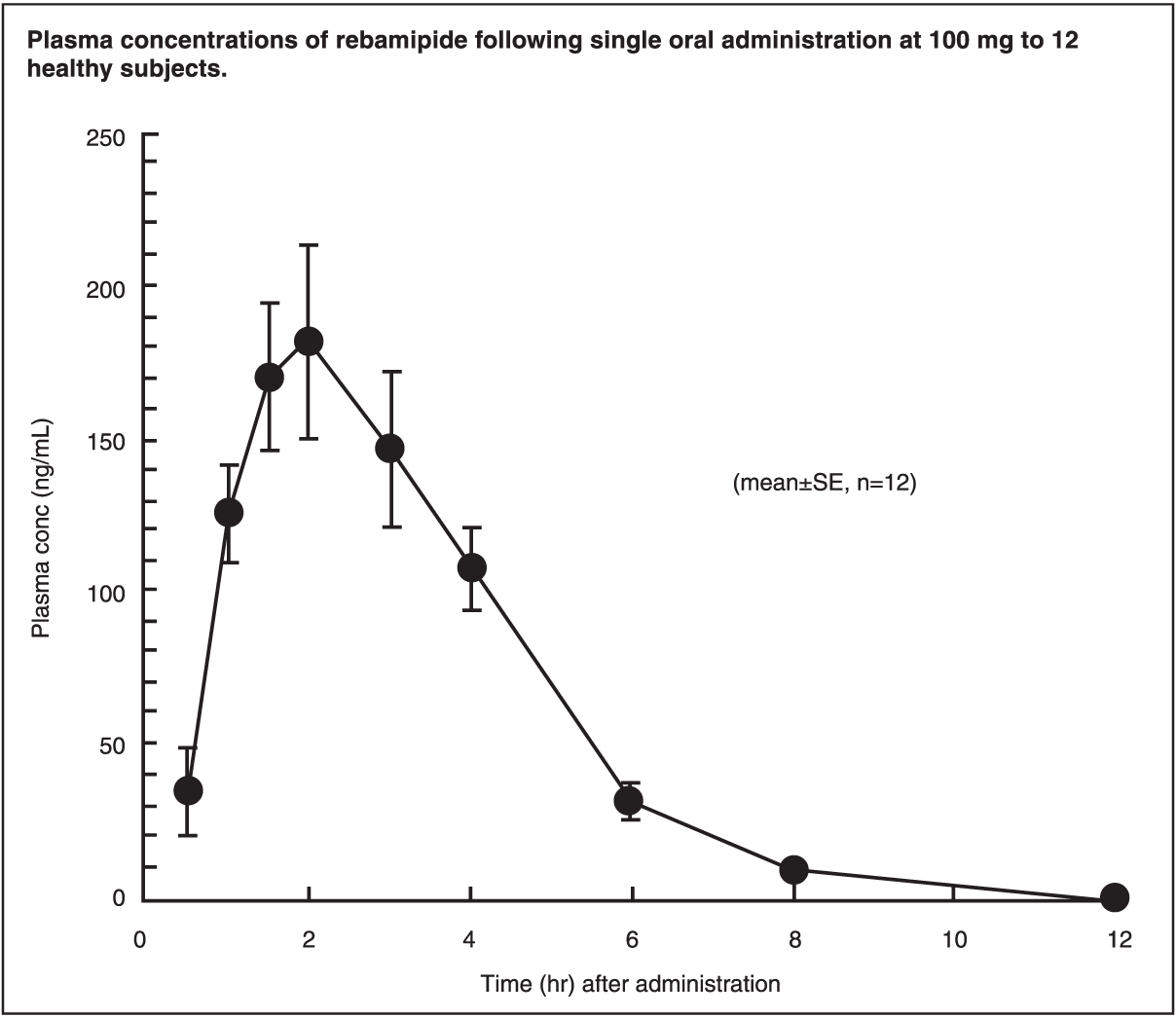
Pharmacokinetics: Plasma Concentrations: Following single oral administration at 100 mg to 12 healthy individuals, plasma concentrations of rebamipide reached the peak level (210 ng/mL) in 2 hrs. The elimination half-life in plasma was about 1.5 hrs.
Repeated administration studies have shown that the drug does not accumulate in humans.
The absorption of rebamipide tended to be slow when the drug was administered orally at a dose of 150 mg to 6 healthy individual subjects after a meal. However, food did not affect bioavailability of rebamipide in humans.
Pharmacokinetic parameters obtained from patients with renal impairment after single oral administration of rebamipide at 100 mg revealed higher plasma concentrations and a longer elimination half-life compared with those in healthy subjects. At steady-state, rebamipide plasma concentrations observed in dialyzed renal patients following repeated administration were very close to the values simulated from single administration. Therefore, the drug was not considered to accumulate. (See figure.)
Pharmacology: Experiments Using Animal Models: Preventive or Healing Effects in Gastric Ulcer Models: Rebamipide inhibited gastric mucosal damage in various experimental rat models of ulcers, including ulcers induced by water-immersion restraint stress, aspirin, indomethacin, histamine, serotonin and pyloric ligation. It also protected the mucosa from damage caused by other ulcerogenic agents that, presumably yield oxygen-free radicals, including mucosal ischemia and reperfusion, administration of platelet activating factor (PAF) or diethyldithiocarbamate (DDC) and administration of indomethacin under stress conditions. The drug promoted healing of gastric ulcers and suppressed recurrence or relapse of ulcers even at 120-140 days after ulcer production in rat acetic acid-induced ulcer model.
Preventive or Healing Effects in Gastritis Models: Rebamipide inhibited the occurrence of taurocholic acid-induced gastritis and promoted healing of mucosal inflammation in a rat gastritis model.
Mechanism of Action: Prostaglandin Increasing Effect: Rebamipide increased the endogenous prostaglandin E2 (PGE2) content in the gastric mucosa in rats. The drug also increased PGE2 and PGI2 content in the gastric juice, as well as the content of 15-keto-13, 14-PGE2, a metabolite of PGE2. In healthy male subjects, the drug again revealed the effect of increasing the PGE2 content in the gastric mucosa and protected the gastric mucosa from injury caused by ethanol loading.
Cytoprotective Effect: Rebamipide exhibited a gastric cytoprotective effect by inhibiting mucosal damage induced by ethanol, strong acid and strong base in rat experiments. The drug also protected gastric epithelial cells in vitro against aspirin- or taurocholic acid-induced injury in cultured cells obtained from rabbit fetuses.
In healthy male subjects, the drug inhibited gastric mucosal injury induced by aspirin, ethanol and HCl-ethanol loading.
Mucus-Increasing Effect: Rebamipide promoted enzyme activity to synthesize high molecular glycoproteins and increased the amounts of gastric surface mucus and soluble mucus in rats. Endogenous PGs are not involved in the increase in soluble mucus.
Mucosal Blood Flow-Increasing Effect: Rebamipide increased gastric mucosal blood flow and improved hemodynamics after blood loss in rats.
Activity on Mucosal Barrier: Rebamipide did not ordinarily affect the gastric transmucosal potential difference in rats, but did inhibit a decrease in the potential difference of ethanol in the stomach of rats.
Activity on Gastric Alkaline Secretion: Rebamipide promotes alkaline secretion in the stomach of rats.
Activity on Mucosal Cell Turnover: Rebamipide activated gastric mucosal cell proliferation and increased the number of epithelial cells in the stomach of rats.
Effect on Gastric Mucous Repair: Rebamipide restored the bile acid- or hydrogen peroxide-induced retardation of artificial wound-repair in cultured rabbit gastric epithelial cells.
Effect on Gastric Secretion: Rebamipide did not alter either basal secretion of gastric juice or stimulatory acid secretion.
Effects on Oxygen-Free Radicals: Rebamipide scavenged hydroxyl radicals and suppressed superoxide production by polymorphonuclear leukocytes. The drug also protected the gastric mucosa from damage by oxygen-free radicals released from neutrophils stimulated by Helicobacter pylori in vitro. The drug inhibited mucosal damage and reduced the content of lipid peroxide in the gastric mucosa of rats treated with indomethacin under stressed conditions.
Effects on Inflammatory Cellular Infiltration in the Gastric Mucosa: Rebamipide prevented inflammatory cellular infiltration in rat models of taurocholic acid-induced gastritis, NSAID-induced gastric mucosal damage and ischemia-perfusion-induced gastric mucosal damage.
Effects on Inflammatory Cytokine Release (Interleukin-8) in the Gastric Mucosa: Rebamipide suppressed the increased production and release of interleukin-8 from human gastric mucosa in the presence of Helicobacter pylori in vitro. The drug also inhibited the activation of NF-KB and suppressed the expression of interleukin-8 mRNA in epithelial cells in vitro. Clinical Studies: Clinical Efficacy in Gastric Ulcer: Mucosta was studied in patients with gastric ulcer using endoscopy for objective drug evaluation. In the final endoscopic assessment, the drug achieved complete healing rate in 60% (200/335) of the patients studied and near-complete healing in 67% (224/335). Clinical usefulness of this drug based on efficacy and safety were demonstrated in a double-blind study. A 6-month follow-up of 67 patients who showed healing at a daily dose of 300 mg revealed that recurrence occurred in only 4 patients (Yulex6%).
Clinical Efficacy in Acute Gastritis and Acute Exacerbation of Chronic Gastritis: Mucosta was studied in patients with acute gastritis or acute exacerbation of chronic gastritis. The drug achieved an 80% (370/461) global efficacy rate in patients evaluated with 76% (351/461) showing moderate or marked improvement. The drug's clinical usefulness can be reproduced in a double-blind study.
Pharmacokinetics: Plasma Concentrations: Following single oral administration at 100 mg to 12 healthy individuals, plasma concentrations of rebamipide reached the peak level (210 ng/mL) in 2 hrs. The elimination half-life in plasma was about 1.5 hrs.
Repeated administration studies have shown that the drug does not accumulate in humans.
The absorption of rebamipide tended to be slow when the drug was administered orally at a dose of 150 mg to 6 healthy individual subjects after a meal. However, food did not affect bioavailability of rebamipide in humans.
Pharmacokinetic parameters obtained from patients with renal impairment after single oral administration of rebamipide at 100 mg revealed higher plasma concentrations and a longer elimination half-life compared with those in healthy subjects. At steady-state, rebamipide plasma concentrations observed in dialyzed renal patients following repeated administration were very close to the values simulated from single administration. Therefore, the drug was not considered to accumulate. (See figure.)
Metabolism: Rebamipide was primarily excreted as the unchanged compound in the urine after single oral administration to healthy adult males at a dose of 600 mg. A metabolite with a hydroxyl group at the 8th position was identified in the urine. However, the excretion of this metabolite was only 0.03% of the administered dose. The enzyme involved in the formation of the metabolite was CYP3A4.
(Note: The usual dosage in adults is 100 mg 3 times daily.)
Excretion: Approximately 10% of the administered dose was excreted in the urine when rebamipide was administered once orally to healthy adult males at 100 mg.
Protein-Binding: Rebamipide at 0.05-5 mcg/mL was added to human plasma in vitro and 98.4-98.6% of the drug was bound to plasma proteins.
Toxicology: Reproductive and Development Toxicity: Reproductive and developmental toxicity studies of rebamipide were conducted using SD rats treated prior to and in the early gestational period at 30-1000 mg/kg/day. SD rats and NZW rabbits were treated during the organogenic period at 30-1000 mg/kg/day, and SD rats were treated during the perinatal and lactational periods at 10-1000 mg/kg/day. The drug exhibited no toxic effects in either parents or F1 offspring in these species.
MedsGo Class
Antacids, Antireflux Agents & Antiulcerants
Features
Dosage
100 mg
Ingredients
- Rebamipide
Packaging
Tablet 1's
Generic Name
Rebamipide
Registration Number
FDA-0237171
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions