Indications/Uses
For isolated or predominant endogenous hypertriglyceridaemia in patient at risk of ischaemic heart disease and or pancreatitis; as a supplement to diet when appropriate and assiduous dietary measures alone are insufficient to produce an adequate response. For the secondary prevention after myocardial infarction.
Dosage/Direction for Use
Post Myocardial Infarction: One capsule daily.
Hypertriglyceridaemia: Initial treatment two capsules daily. If adequate response is not obtained, the dose may be increased to four capsule daily.
The capsules may be taken with food to avoid gastrointestinal disturbances.
There is no information regarding the use of Omacor in children and adolescents, in elderly patients over 70 years of age, or in patients with hepatic impairment, and only limited information regarding the use in patients with renal impairment.
Hypertriglyceridaemia: Initial treatment two capsules daily. If adequate response is not obtained, the dose may be increased to four capsule daily.
The capsules may be taken with food to avoid gastrointestinal disturbances.
There is no information regarding the use of Omacor in children and adolescents, in elderly patients over 70 years of age, or in patients with hepatic impairment, and only limited information regarding the use in patients with renal impairment.
Overdosage
There are no special recommendations for overdosage with Omacor. Treatment should be symptomatic.
Administration
Should be taken with food: Take w/ food to avoid GI disturbances.
Contraindications
Hypersensitivity to the active substance, to soya (including soya milk, soya beans) or to any of the excipients.
Special Precautions
During treatment with Omacor there is a fall in thromboxane A2 production. No significant effect has been observed on the other coagulation factors. Some studies with omega-3-acids demonstrated a prolongation of bleeding time, but the bleeding time reported in these studies has not exceeded normal limits and did not produce clinically significant bleeding episodes.
Clinical studies have not been done to thoroughly examine the combined effects of Omacor and concomitant anticoagulants. Patients receiving treatment with Omacor and an anticoagulant or other drug affecting coagulation (eg, acetylsalicylic acid, warfarin and coumarin) should be monitored periodically, and the dosage of anticoagulant therapy adjusted if necessary.
It is recommended that routine monitoring of the entire lipid profile is undertaken. As a possible rise in LDL-C has been shown in some studies with intake of Omacor 4 g/day (see Pharmacology: Pharmacodynamics under Clinical Trials), LDL-C should therefore be monitored on a regular basis, especially in patients with type IV and V dyslipidaemia.
Omacor is not recommended as monotherapy in Type IIb dyslipidaemia. Statins are to be used as first line treatment with Omacor indicated as add-on therapy when control of the triglyceride levels is required.
Hepatic Impairment: Regular monitoring of hepatic function (especially ALT - see Adverse Reactions, and AST) is required in patients with hepatic impairment, in particular with the higher dosage of 4 g per day.
Use in children: In the absence of efficacy and safety data, the use of this medication in children is not recommended.
Effects on Fertility: No adverse effects on fertility were observed in a rat fertility study at oral doses of up to 2,000 mg/kg/day (35 times the human dose of 4 g/day on a mg/kg basis).
Carcinogenicity: There was no evidence of a carcinogenic effect of Omacor from the carcinogenicity studies in rats and mice at oral doses of up to 2,000 mg/kg/day (35 times the human dose of 4 g/day on a mg/kg basis).
Genotoxicity: There was no clear evidence of a genotoxic effect of Omacor from the genotoxicity studies conducted (Ames test in Salmonella typhinurium, gene mutation at the HGPRT locus in Chinese hamster V79 cells, chromosome aberration study in cultured human lymphocytes and in vivo mouse micronucleus test).
Use in Pregnancy: Category B1. There are no adequate data from the use of Omacor in pregnant women. The potential risk for humans is unknown. Therefore Omacor should not be used during pregnancy unless clearly necessary.
Use in Lactation: There are no data on the excretion of Omacor components in human milk. Because many drugs are excreted in human milk, caution should be exercised when Omacor is administered to a woman who is breastfeeding.
Clinical studies have not been done to thoroughly examine the combined effects of Omacor and concomitant anticoagulants. Patients receiving treatment with Omacor and an anticoagulant or other drug affecting coagulation (eg, acetylsalicylic acid, warfarin and coumarin) should be monitored periodically, and the dosage of anticoagulant therapy adjusted if necessary.
It is recommended that routine monitoring of the entire lipid profile is undertaken. As a possible rise in LDL-C has been shown in some studies with intake of Omacor 4 g/day (see Pharmacology: Pharmacodynamics under Clinical Trials), LDL-C should therefore be monitored on a regular basis, especially in patients with type IV and V dyslipidaemia.
Omacor is not recommended as monotherapy in Type IIb dyslipidaemia. Statins are to be used as first line treatment with Omacor indicated as add-on therapy when control of the triglyceride levels is required.
Hepatic Impairment: Regular monitoring of hepatic function (especially ALT - see Adverse Reactions, and AST) is required in patients with hepatic impairment, in particular with the higher dosage of 4 g per day.
Use in children: In the absence of efficacy and safety data, the use of this medication in children is not recommended.
Effects on Fertility: No adverse effects on fertility were observed in a rat fertility study at oral doses of up to 2,000 mg/kg/day (35 times the human dose of 4 g/day on a mg/kg basis).
Carcinogenicity: There was no evidence of a carcinogenic effect of Omacor from the carcinogenicity studies in rats and mice at oral doses of up to 2,000 mg/kg/day (35 times the human dose of 4 g/day on a mg/kg basis).
Genotoxicity: There was no clear evidence of a genotoxic effect of Omacor from the genotoxicity studies conducted (Ames test in Salmonella typhinurium, gene mutation at the HGPRT locus in Chinese hamster V79 cells, chromosome aberration study in cultured human lymphocytes and in vivo mouse micronucleus test).
Use in Pregnancy: Category B1. There are no adequate data from the use of Omacor in pregnant women. The potential risk for humans is unknown. Therefore Omacor should not be used during pregnancy unless clearly necessary.
Use in Lactation: There are no data on the excretion of Omacor components in human milk. Because many drugs are excreted in human milk, caution should be exercised when Omacor is administered to a woman who is breastfeeding.
Adverse Reactions
Post Myocardial Infarction: From the GISSI-Prevenzione study.
Adverse effects were reported as a reason for discontinuation of the therapy for 3.8% of the patients in the Omacor groups, and in 2.1% in the vitamin E-groups. Overall, gastrointestinal disturbances and nausea were the most reported adverse effects, 4.9% and 1.4% of the Omacor recipients, and 2.9% and 0.4% of vitamin E recipients.
Hypertriglyceridaemia: In all subjects (655) treated with Omacor for hypertriglyceridaemia, the following results were seen: Adverse events (AEs) occurred in approximately 30% of subjects; Only 11 specific AEs occurred at a rate greater than 1%; The most common treatment-emergent AEs were eructation (4.4%) and taste perversion (4.1%); Treatment emergent serious adverse events occurred in 2.4% of subjects; Four subjects died.
The 8 pivotal trials showed similar safety profiles.
The only potentially drug-related laboratory abnormally was mild elevation in alanine aminotransferase (ALT) levels, without concurrent elevation in aspartate aminotransferase (ASD) levels.
A slight, but significant, prolongation of bleeding time has been observed without any reports of bleeding problems during clinical trials with Omacor alone.
The following table summarizes the treatment-emergent adverse events experienced by subjects from placebo controlled studies in hypertriglyceridaemia, using Omacor 4 g per day (see Pharmacology: Pharmacodynamics: Clinical Trials under Actions). (See Table 6).
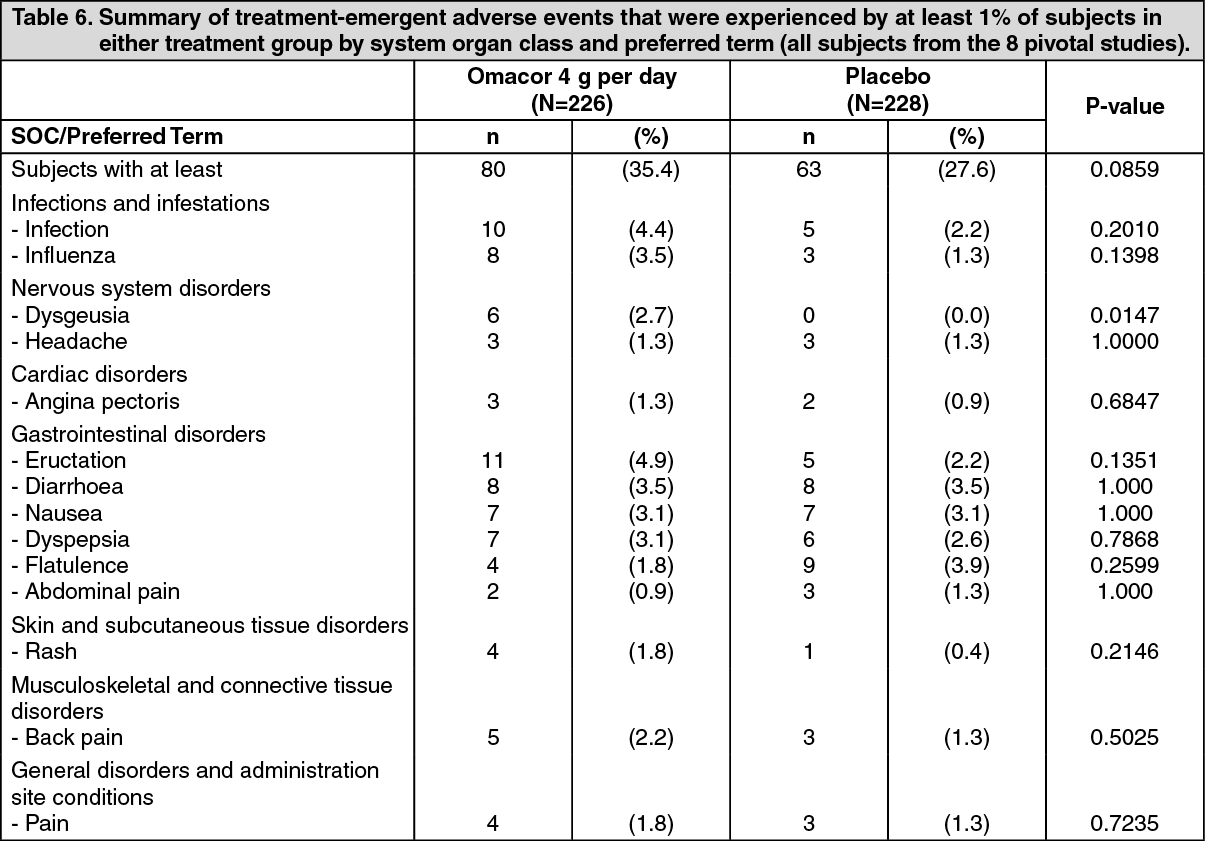
Adverse events according to System Organ Class: The following list presents the frequencies of study related adverse events, observed both in post myocardial infarction and in hypertriglyceridaemia.
Immune system disorders: Rare: hypersensitivity.
Metabolism and nutrition disorders: Uncommon: hyperglycaemia, gout.
Nervous System disorders: Uncommon: dizziness, dysgeusia, headache.
Vascular disorders: Uncommon: hypotension.
Respiratory, thoracic and mediastinal disorders: Uncommon: epistaxis.
Gastrointestinal disorders: Common: gastrointestinal disorders (including abdominal distension, abdominal pain, constipation, diarrhoea, dyspepsia, flatulence, eructation, gastro-oesophageal reflux disease, nausea or vomiting).
Uncommon: gastrointestinal hemorrhage.
Hepatobiliary disorders: Rare: liver disorders (including transaminases increased, alanine aminotransferase increased and aspartate aminotransferase increased).
Skin and subcutaneous tissue disorders: Uncommon: rash.
Rare: urticaria.
Adverse effects were reported as a reason for discontinuation of the therapy for 3.8% of the patients in the Omacor groups, and in 2.1% in the vitamin E-groups. Overall, gastrointestinal disturbances and nausea were the most reported adverse effects, 4.9% and 1.4% of the Omacor recipients, and 2.9% and 0.4% of vitamin E recipients.
Hypertriglyceridaemia: In all subjects (655) treated with Omacor for hypertriglyceridaemia, the following results were seen: Adverse events (AEs) occurred in approximately 30% of subjects; Only 11 specific AEs occurred at a rate greater than 1%; The most common treatment-emergent AEs were eructation (4.4%) and taste perversion (4.1%); Treatment emergent serious adverse events occurred in 2.4% of subjects; Four subjects died.
The 8 pivotal trials showed similar safety profiles.
The only potentially drug-related laboratory abnormally was mild elevation in alanine aminotransferase (ALT) levels, without concurrent elevation in aspartate aminotransferase (ASD) levels.
A slight, but significant, prolongation of bleeding time has been observed without any reports of bleeding problems during clinical trials with Omacor alone.
The following table summarizes the treatment-emergent adverse events experienced by subjects from placebo controlled studies in hypertriglyceridaemia, using Omacor 4 g per day (see Pharmacology: Pharmacodynamics: Clinical Trials under Actions). (See Table 6).

Adverse events according to System Organ Class: The following list presents the frequencies of study related adverse events, observed both in post myocardial infarction and in hypertriglyceridaemia.
Immune system disorders: Rare: hypersensitivity.
Metabolism and nutrition disorders: Uncommon: hyperglycaemia, gout.
Nervous System disorders: Uncommon: dizziness, dysgeusia, headache.
Vascular disorders: Uncommon: hypotension.
Respiratory, thoracic and mediastinal disorders: Uncommon: epistaxis.
Gastrointestinal disorders: Common: gastrointestinal disorders (including abdominal distension, abdominal pain, constipation, diarrhoea, dyspepsia, flatulence, eructation, gastro-oesophageal reflux disease, nausea or vomiting).
Uncommon: gastrointestinal hemorrhage.
Hepatobiliary disorders: Rare: liver disorders (including transaminases increased, alanine aminotransferase increased and aspartate aminotransferase increased).
Skin and subcutaneous tissue disorders: Uncommon: rash.
Rare: urticaria.
Drug Interactions
Increased bleeding time has been seen when Omacor is given in conjunction with acetylsalicylic acid and warfarin, but without haemorrhagic complications (see Precautions).
Acetylsalicylic acid: Patients should be informed about potential increased bleeding time.
Warfarin and coumarin: The prothrombin time/international normalised ratio (PT/INR) must be monitored during combination treatment with Omacor among patients receiving blood-thinning therapy, and when treatment with Omacor is discontinued.
Statins: Omacor 4 g has been administered with simvastatin 80 mg under fasting conditions to 24 healthy volunteers in a two 14-days period drug-drug interaction study. Results of this study demonstrated that at steady state, the co-administration of Omacor capsules with simvastatin did not appear to affect the pharmacokinetics of simvastatin tablets. The combination appeared to be well tolerated.
Acetylsalicylic acid: Patients should be informed about potential increased bleeding time.
Warfarin and coumarin: The prothrombin time/international normalised ratio (PT/INR) must be monitored during combination treatment with Omacor among patients receiving blood-thinning therapy, and when treatment with Omacor is discontinued.
Statins: Omacor 4 g has been administered with simvastatin 80 mg under fasting conditions to 24 healthy volunteers in a two 14-days period drug-drug interaction study. Results of this study demonstrated that at steady state, the co-administration of Omacor capsules with simvastatin did not appear to affect the pharmacokinetics of simvastatin tablets. The combination appeared to be well tolerated.
Storage
Store at room temperatures not exceeding 30°C.
Action
Pharmacology: Pharmacodynamics: The omega-3 series polyunsaturated fatty acids (OFA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are essential fatty acids. They are essential nutrients that cannot be synthesized by the human body in sufficient amounts and have to be obtained in the diet.
Like all fatty acids, omega-3 fatty acids are used to provide energy and are stored in adipose tissue; small amounts are incorporated into cell membranes as well.
Omacor is active on the plasma lipids by lowering triglyceride levels as a result of a fall in VLDL (very low density lipoprotein), and the substance is also active on homeostasis and blood pressure.
The mechanism of action of Omacor in lowering plasma triglycerides (TG) is not completely understood. Potential mechanisms of action include inhibition of acyl CoA:1,2-diacylglycerol acyltransferase, increased mitochondrial and paroxysmal B-oxidation of fatty acids in the liver and decreased lipogenesis in the liver. Omacor may reduce the synthesis of TG in the liver because EPA and DHA are poor substrates for the enzymes responsible for TG synthesis, and EPA and DHA inhibit esterification of other fatty acids.
The exact mechanism of action in the secondary prevention after a myocardial infarction is not yet known and is currently being evaluated. Several studies have been performed with omega-3 formulations showing that OFA induce several beneficial changes in traditional risk factors for Coronary Heart Disease (CHD) which make omega-3 acids attractive in the prophylaxis and treatment of cardiovascular diseases.
Omacor increases low density lipoproteins (LDL) cholesterol in some patients with hypertriglyceridaemia.
A small rise in high-density lipoproteins (HDL) cholesterol has also been observed however it is significantly smaller than seen after fibrates, and is not consistent across this population subset.
There is no strong evidence that lowering the triglycerides reduces the risk of ischaemic heart disease.
During treatment with Omacor a decrease in thromboxane A2 production has been observed and a slight increase in bleeding time (particularly with the higher doses, 4 g per day). No significant effect has been observed on the other coagulation factors (see Precautions).
Omacor has been shown to cause a significant reduction in blood pressure.
Clinical trials: Post Myocardial Infarction (MI): GISSI-Prevenzione study: A multi-centre, randomized, open-label study performed in Italy, enrolled 11324 patients, with recent MI (<3 months; [50% within 16 days and 72% within 30days]) and receiving recommended preventive treatments associated with a Mediterranean diet: antiplatelet drugs, mainly aspirin (overall 82.8% at 42 months), beta-blockers (38.5% at 42 months) and ACE inhibitors (39.0% at 42 months). Since statins were not supported by definitive data on efficacy when the GISSI-Prevenzione trial was started in 1993, only 4.7% of the patients received a statin at baseline. Most of the patients were normolipidaemic (mean value of total cholesterol (TC) was 211.6 mg/dL (5.459 mmol/L), mean value of serum TG was 161.9 mg/dL (1.846 mmol/L) at baseline).
A relatively large proportion of the patients were aged > 70 years. The only exclusion criterion was any condition associated with a poor short-term prognosis (including, but not limited to, severe congestive heart failure and cancer). Patients were randomised to Omacor (N=2,836), vitamin E (N=2,830), Omacor and Vitamin E (N=2,830) or no treatment (N=2,828). The dose of Omacor was 1 g daily, and vitamin E was 300 mg daily. Mean duration of treatment was 3.5 years.
The analysis in the GISSI-Prevenzione trial was performed for the intention-to-treat (ITT) sample and according to two strategies defined in the protocol: An analysis of efficacy of the combined two Omacor treated groups compared to the combined two treatment groups without Omacor, and efficacy of the combined two vitamin E supplements treated groups compared with the combination of the two treatment groups with no vitamin E. This is the two-way analysis.
An analysis of efficacy of each of the treatment groups: Omacor, vitamin E supplements, and the combination versus the control group, as well as comparisons between the combination versus the Omacor only group and the vitamin E only group. This is the four way analysis.
The data were analysed by Kaplan-Meier-survival curves and the log-rank test. In order to further quantify treatment effects, the relative risks and associated confidence intervals were assessed using Cox's proportional hazards models adjusted for the confounding effects of relevant prognostic indicators.
In Table 1 hereafter the main results of two-way analysis are summarized for the main endpoints and selected secondary endpoints. Results of the log-rank test as well as the relative risk together with the 95% confidence interval are presented for the Omacor group and the control group.
For this two-way analysis a 10% relative decrease in risk and a 1.3% absolute decrease in risk are observed for the combined endpoint of death, non-fatal MI and nonfatal stroke (Number Needed to Treat NNT=77). The log-rank test was significant with a p-value of 0.048.
The relative and absolute risk decreases for the second combined endpoint cardiovascular death, nonfatal MI and nonfatal stroke were respectively 11% and 1.1% (Number needed to treat NNT=91). The log-rank test result of p=0.053 was comparable to the p-value of the first combined endpoint; however, it was not significant.
Analysis of the individual components of the main endpoint showed significant differences between the two treatment groups for total mortality (p=0.016), cardiovascular death (p=0.019), coronary death (p=0.016) and sudden death (p=0.011). There was no difference across the treatment groups for non-fatal cardiovascular events and other deaths. (See Table 1).
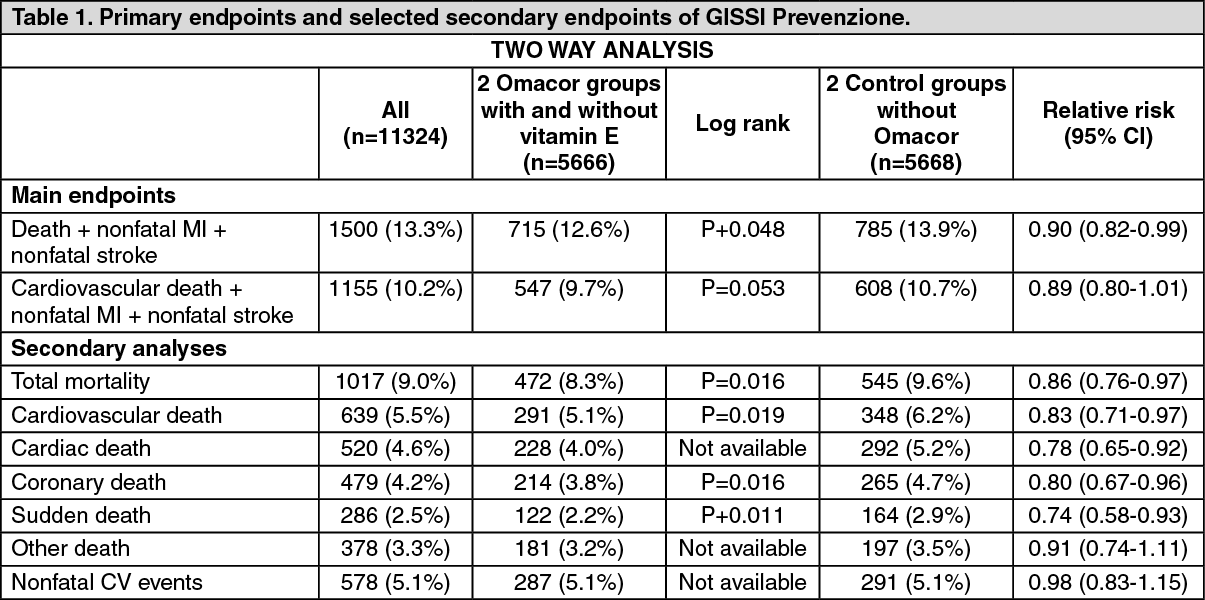
In Table 2 hereafter the results of the four-way analysis are presented for the main endpoints and selected secondary endpoints. Results of the log-rank test as well as the relative risk together with the 95% confidence interval are presented for only the Omacor group and the control group.
For this four-way analysis a 15% relative decrease in risk and a 2.3% absolute decrease in risk are observed for the combined endpoint of death, non-fatal Ml and nonfatal stroke (Number Needed to Treat NNT=43). The log-rank test was significant with a p-value of 0.023.
The relative and absolute risk decreases for the second combined endpoint cardiovascular death, nonfatal MI and nonfatal stroke were respectively 20% and 1.2% (Number Needed to Treat NNT=83). The log-rank test result was highly significant with a p-value of 0.008. Analysis of the individual components of the main endpoint showed significant differences between the two treatment groups for total mortality (p=0.009), cardiovascular death (p=0,001), coronary death (p=0.001) and sudden death (p=0.0004). There was no difference across the treatment groups for non-fatal cardiovascular events (relative risk of 0.96 and 95% CI of 0.76-1.21) and other deaths with a relative risk of 0.99 and 95% CI of 0.75-1.30. (See Table 2).
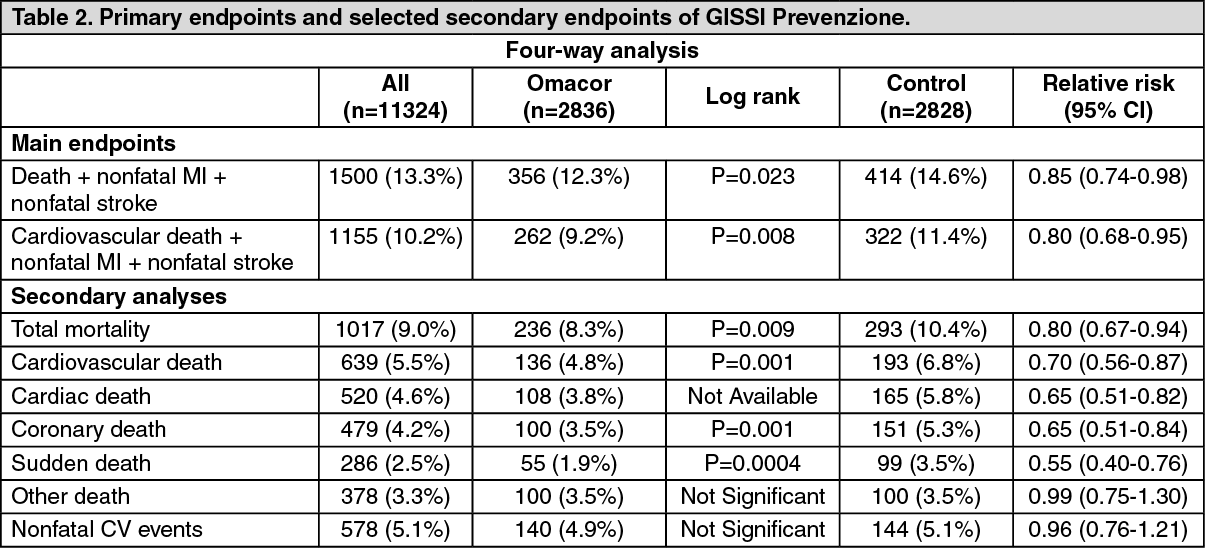
Referring to the Lancet publication of the GISSI-Prevenzione study (1999), the first primary combined endpoint (death, non-fatal MI and non-fatal stroke) reached statistical significance for the overall efficacy of Omacor plus vitamin E.
For the second primary combined endpoint (cardiovascular death, non-fatal MI and non-fatal stroke), neither the two-way analysis for the Omacor effect (Table 3 in the publication) nor the overall efficacy profile of Omacor plus vitamin E treatment (Table 5 in the publication) reached statistical significance, even so the risk reduction trend is clear.
The risk reductions for the Omacor treatment group were 20% in the four-way analysis (statistically significant) and 11% in the two-way analysis (non-significant), accordingly. For the combined Omacor plus vitamin E treatment group, the analysis showed a risk reduction of 12%, thus demonstrating a small additional risk reduction of the combined treatment compared to the two-way analysis of the n-3 PUFA group (see the graph below).
In the analysis of this latter overall efficacy profile, the investigators compared the large Omacor group (5666 patients), including those taking a combination of Omacor and vitamin E, to the small "clean" control group of 2828 patients, resulting in a broader confidence interval than two-way analysis where the control group also included the vitamin E-group (N=5658). (See figure).
At the end of the study 28.5% of the patients receiving Omacor and 262% of the patients receiving vitamin E has discontinued treatment thirteen patients were lost to follow-up.
The investigators also assessed the time course of the benefit of Omacor. Patients allocated to Omacor had a significantly lower mortality even after 3 months of treatment (1.1% versus 1.6%, relative risk [RR] 0.59, confidence interval [Cl] 0.36 to 0.97; p.,0.037). The reduction in sudden cardiac death was almost significant at only 3 months, accounting for up to 57% of the overall mortality benefit (0.5% versus 0.7%; RR 0.44; P=0.048). The benefit on sudden cardiac death became significant at 4 months (2.0% versus 2.7%; RR 0.55, 95% CI 0.39 to 0.77; p=0.0006).
Hypertriglyceridaemia: There have been eight double-blind, parallel group, placebo-controlled studies in hypertriglyceridaemia, using Omacor 4 g per day. These eight studies are the pivotal studies. These studies included seven individual studies and one part of a study that evaluated Omacor 2 g, 4 g, 8 g, and placebo treatment arms.
The duration of the eight pivotal studies was short term (maximum 12 weeks). Numerous studies in patients with hypertriglyceridaemia have been conducted with Omacor, with variable designs: double-blind studies, placebo-controlled studies, randomized studies, open studies and long term studies (up to 24 months). Omacor at doses of 4 g per day consistently and significantly reduced triglycerides levels compared to placebo. The studies have shown that the reductions were maintained for up 24 months after treatment. (See Table 3).
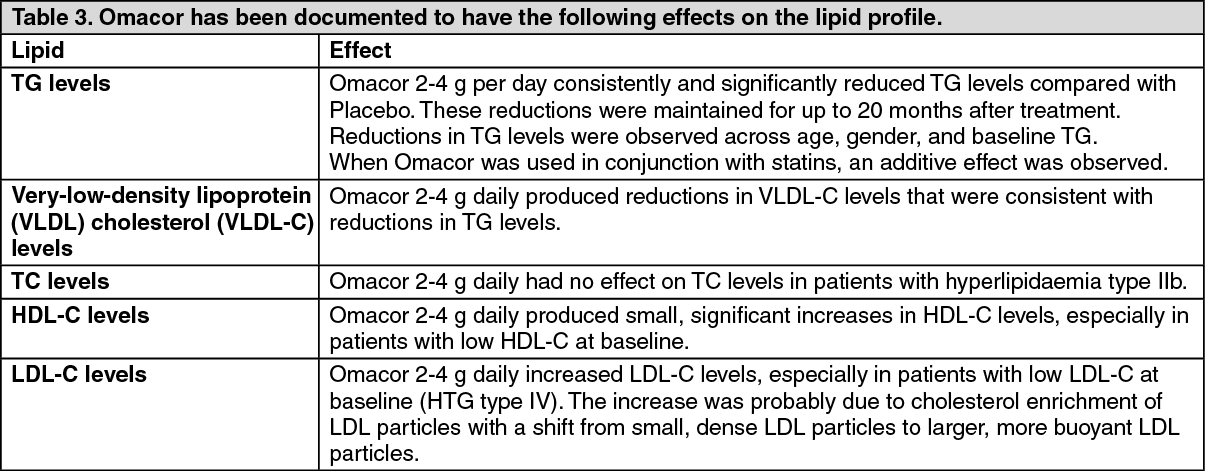
The following table summarizes the median percent changes in lipid parameters from baseline in the overall population, and in patients with Types IIb, IV and V dyslipidaemia. (See Table 4).
Remarks: The documented number of patients enrolled in clinical trials with Type 1 dyslipidaemia is very limited and no studies were designed to especially investigate the effect of Omacor in these patients. Type HI dyslipidaemic patients are homozygotes for ApoE, and genotyping of patients was only performed in one study (K85-95011). More Type III dyslipidemic patients may have been therefore enrolled in clinical studies without being verified as such.
There is no reason to believe that Type HI dyslipidaemic patients do not respond to Omacor.
One of the pivotal clinical trials in patients with type IV and V (K85-95009 study) demonstrated a mean LDL-C increase of 42.6% with Omacor 4 g day. 67% of the patients in the study experienced increases in LDL-C, and the increases observed were in the range of 6%-110%. However, mean LDL-C concentrations at the end of the study were still only equal to 2.69 mmol/L (104 mg/dL). For the majority of these patients (40 of 42 with no history of coronary disease) this is still below their target LDL-C levels. Only equal to 2.69 mmol/L (104 mg/dL).
In clinical trials on patients with Type Hb dyslipidaemia mean LDL-C is unchanged or slightly increased (maximum 8.6%) with Omacor treatment. In studies with concomitant treatment of Omacor and a statin no significant increase in LDL-C has been observed with Omacor.
The cholesterol enrichment of LDL particles appears to happen in conjunction with a marked reduction in VLDL-C.
Studies also demonstrate a shift from small, dense LDL particles to larger, more buoyant LDL particles, indicating a shift towards less atherogenic lipoprotein particles.
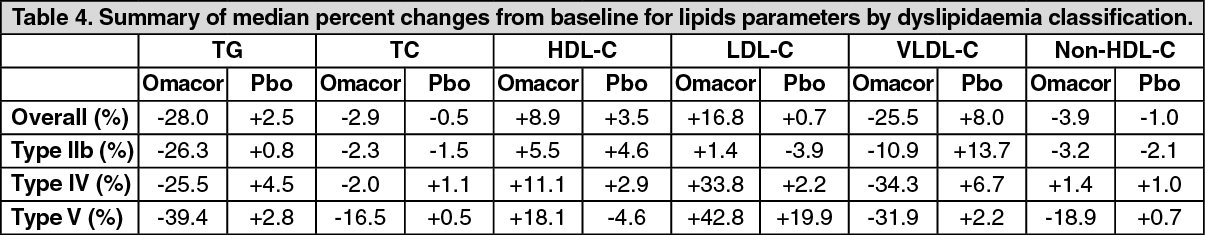
Consistent with the overall population (see Table 5 hereafter), subjects in each baseline triglycerides level category in the Omacor 4 g treatment group had significantly larger mean absolute and relative changes in triglycerides levels compared with those in the placebo treatment group.
For the subjects who received Omacor 4 g per day, those with higher baseline levels (TG 500-749 mg/dL and 3750 mg/dL [5.65-8.46 mmol/L, and 8.47 mmol/L]) had greater reductions in triglycerides levels, and therefore were more likely to exhibit a better response to Omacor. (See Table 5).
A number of studies have been conducted to evaluate the effect of concomitant use of Omacor with widely used statins (simvastatin, atorvastatin). The studies have been carried out in patients with elevated serum triglycerides receiving statin therapy. The results of the studies demonstrate that the combined treatment increases the efficacy in lowering triglycerides. In these studies little or no effect on LDL-C has been observed and no significant safety issues have been raised.
Pharmacokinetics: The hydrolysis of omega-3 ethyl esters by esterases in the intestine is complete and rapid. After absorption, OFA are metabolised by multiple pathways that are not highly predictable. Animal pharmacokinetic studies have shown that there is no systemic exposure of the ethyl esters. Due to this complicated process, it is not possible to conduct standard bioavailability studies, and consequently, to measure meaningful values for Cmax, Tmax, AUC, etc. for Omacor.
The levels of EPA and DHA do increase on ingestion of Omacor, although in a less than dose proportional manner.
The absorption of Omacor has been determined by measuring the increase of EPA and DHA in plasma serum phospholipids after dosing. Significant, dose-dependent increases in serum phospholipids EPA content were seen, while increase in DHA incorporation were less marked and not dose dependent. Uptake of EPA and DHA into plasma/serum phospholipids in subjects treated with Omacor was also independent of gender, age, and hypertensive status. Concomitant ingestion of another unsaturated fatty acid, olive oil, did not affect absorption of omega-3 fatty acids from Omacor.
During and after absorption there are three main pathways for the metabolism of the omega-3 fatty acids: The fatty acids are first transported to the liver where they are incorporated into various categories of lipoproteins and then channeled to the peripheral lipids stores.
The cell membrane phospholipids are replaced by lipoprotein phospholipids and the fatty acids can then act as precursors for various eicosanoids.
The majority is oxidized to meet energy requirements.
The concentration of omega-3 fatty acids, EPA and DHA, in the plasma phospholipids corresponds to the EPA and DHA incorporated into the cell membranes.
Like all fatty acids, omega-3 fatty acids are used to provide energy and are stored in adipose tissue; small amounts are incorporated into cell membranes as well.
Omacor is active on the plasma lipids by lowering triglyceride levels as a result of a fall in VLDL (very low density lipoprotein), and the substance is also active on homeostasis and blood pressure.
The mechanism of action of Omacor in lowering plasma triglycerides (TG) is not completely understood. Potential mechanisms of action include inhibition of acyl CoA:1,2-diacylglycerol acyltransferase, increased mitochondrial and paroxysmal B-oxidation of fatty acids in the liver and decreased lipogenesis in the liver. Omacor may reduce the synthesis of TG in the liver because EPA and DHA are poor substrates for the enzymes responsible for TG synthesis, and EPA and DHA inhibit esterification of other fatty acids.
The exact mechanism of action in the secondary prevention after a myocardial infarction is not yet known and is currently being evaluated. Several studies have been performed with omega-3 formulations showing that OFA induce several beneficial changes in traditional risk factors for Coronary Heart Disease (CHD) which make omega-3 acids attractive in the prophylaxis and treatment of cardiovascular diseases.
Omacor increases low density lipoproteins (LDL) cholesterol in some patients with hypertriglyceridaemia.
A small rise in high-density lipoproteins (HDL) cholesterol has also been observed however it is significantly smaller than seen after fibrates, and is not consistent across this population subset.
There is no strong evidence that lowering the triglycerides reduces the risk of ischaemic heart disease.
During treatment with Omacor a decrease in thromboxane A2 production has been observed and a slight increase in bleeding time (particularly with the higher doses, 4 g per day). No significant effect has been observed on the other coagulation factors (see Precautions).
Omacor has been shown to cause a significant reduction in blood pressure.
Clinical trials: Post Myocardial Infarction (MI): GISSI-Prevenzione study: A multi-centre, randomized, open-label study performed in Italy, enrolled 11324 patients, with recent MI (<3 months; [50% within 16 days and 72% within 30days]) and receiving recommended preventive treatments associated with a Mediterranean diet: antiplatelet drugs, mainly aspirin (overall 82.8% at 42 months), beta-blockers (38.5% at 42 months) and ACE inhibitors (39.0% at 42 months). Since statins were not supported by definitive data on efficacy when the GISSI-Prevenzione trial was started in 1993, only 4.7% of the patients received a statin at baseline. Most of the patients were normolipidaemic (mean value of total cholesterol (TC) was 211.6 mg/dL (5.459 mmol/L), mean value of serum TG was 161.9 mg/dL (1.846 mmol/L) at baseline).
A relatively large proportion of the patients were aged > 70 years. The only exclusion criterion was any condition associated with a poor short-term prognosis (including, but not limited to, severe congestive heart failure and cancer). Patients were randomised to Omacor (N=2,836), vitamin E (N=2,830), Omacor and Vitamin E (N=2,830) or no treatment (N=2,828). The dose of Omacor was 1 g daily, and vitamin E was 300 mg daily. Mean duration of treatment was 3.5 years.
The analysis in the GISSI-Prevenzione trial was performed for the intention-to-treat (ITT) sample and according to two strategies defined in the protocol: An analysis of efficacy of the combined two Omacor treated groups compared to the combined two treatment groups without Omacor, and efficacy of the combined two vitamin E supplements treated groups compared with the combination of the two treatment groups with no vitamin E. This is the two-way analysis.
An analysis of efficacy of each of the treatment groups: Omacor, vitamin E supplements, and the combination versus the control group, as well as comparisons between the combination versus the Omacor only group and the vitamin E only group. This is the four way analysis.
The data were analysed by Kaplan-Meier-survival curves and the log-rank test. In order to further quantify treatment effects, the relative risks and associated confidence intervals were assessed using Cox's proportional hazards models adjusted for the confounding effects of relevant prognostic indicators.
In Table 1 hereafter the main results of two-way analysis are summarized for the main endpoints and selected secondary endpoints. Results of the log-rank test as well as the relative risk together with the 95% confidence interval are presented for the Omacor group and the control group.
For this two-way analysis a 10% relative decrease in risk and a 1.3% absolute decrease in risk are observed for the combined endpoint of death, non-fatal MI and nonfatal stroke (Number Needed to Treat NNT=77). The log-rank test was significant with a p-value of 0.048.
The relative and absolute risk decreases for the second combined endpoint cardiovascular death, nonfatal MI and nonfatal stroke were respectively 11% and 1.1% (Number needed to treat NNT=91). The log-rank test result of p=0.053 was comparable to the p-value of the first combined endpoint; however, it was not significant.
Analysis of the individual components of the main endpoint showed significant differences between the two treatment groups for total mortality (p=0.016), cardiovascular death (p=0.019), coronary death (p=0.016) and sudden death (p=0.011). There was no difference across the treatment groups for non-fatal cardiovascular events and other deaths. (See Table 1).

In Table 2 hereafter the results of the four-way analysis are presented for the main endpoints and selected secondary endpoints. Results of the log-rank test as well as the relative risk together with the 95% confidence interval are presented for only the Omacor group and the control group.
For this four-way analysis a 15% relative decrease in risk and a 2.3% absolute decrease in risk are observed for the combined endpoint of death, non-fatal Ml and nonfatal stroke (Number Needed to Treat NNT=43). The log-rank test was significant with a p-value of 0.023.
The relative and absolute risk decreases for the second combined endpoint cardiovascular death, nonfatal MI and nonfatal stroke were respectively 20% and 1.2% (Number Needed to Treat NNT=83). The log-rank test result was highly significant with a p-value of 0.008. Analysis of the individual components of the main endpoint showed significant differences between the two treatment groups for total mortality (p=0.009), cardiovascular death (p=0,001), coronary death (p=0.001) and sudden death (p=0.0004). There was no difference across the treatment groups for non-fatal cardiovascular events (relative risk of 0.96 and 95% CI of 0.76-1.21) and other deaths with a relative risk of 0.99 and 95% CI of 0.75-1.30. (See Table 2).

Referring to the Lancet publication of the GISSI-Prevenzione study (1999), the first primary combined endpoint (death, non-fatal MI and non-fatal stroke) reached statistical significance for the overall efficacy of Omacor plus vitamin E.
For the second primary combined endpoint (cardiovascular death, non-fatal MI and non-fatal stroke), neither the two-way analysis for the Omacor effect (Table 3 in the publication) nor the overall efficacy profile of Omacor plus vitamin E treatment (Table 5 in the publication) reached statistical significance, even so the risk reduction trend is clear.
The risk reductions for the Omacor treatment group were 20% in the four-way analysis (statistically significant) and 11% in the two-way analysis (non-significant), accordingly. For the combined Omacor plus vitamin E treatment group, the analysis showed a risk reduction of 12%, thus demonstrating a small additional risk reduction of the combined treatment compared to the two-way analysis of the n-3 PUFA group (see the graph below).
In the analysis of this latter overall efficacy profile, the investigators compared the large Omacor group (5666 patients), including those taking a combination of Omacor and vitamin E, to the small "clean" control group of 2828 patients, resulting in a broader confidence interval than two-way analysis where the control group also included the vitamin E-group (N=5658). (See figure).

At the end of the study 28.5% of the patients receiving Omacor and 262% of the patients receiving vitamin E has discontinued treatment thirteen patients were lost to follow-up.
The investigators also assessed the time course of the benefit of Omacor. Patients allocated to Omacor had a significantly lower mortality even after 3 months of treatment (1.1% versus 1.6%, relative risk [RR] 0.59, confidence interval [Cl] 0.36 to 0.97; p.,0.037). The reduction in sudden cardiac death was almost significant at only 3 months, accounting for up to 57% of the overall mortality benefit (0.5% versus 0.7%; RR 0.44; P=0.048). The benefit on sudden cardiac death became significant at 4 months (2.0% versus 2.7%; RR 0.55, 95% CI 0.39 to 0.77; p=0.0006).
Hypertriglyceridaemia: There have been eight double-blind, parallel group, placebo-controlled studies in hypertriglyceridaemia, using Omacor 4 g per day. These eight studies are the pivotal studies. These studies included seven individual studies and one part of a study that evaluated Omacor 2 g, 4 g, 8 g, and placebo treatment arms.
The duration of the eight pivotal studies was short term (maximum 12 weeks). Numerous studies in patients with hypertriglyceridaemia have been conducted with Omacor, with variable designs: double-blind studies, placebo-controlled studies, randomized studies, open studies and long term studies (up to 24 months). Omacor at doses of 4 g per day consistently and significantly reduced triglycerides levels compared to placebo. The studies have shown that the reductions were maintained for up 24 months after treatment. (See Table 3).

The following table summarizes the median percent changes in lipid parameters from baseline in the overall population, and in patients with Types IIb, IV and V dyslipidaemia. (See Table 4).

Remarks: The documented number of patients enrolled in clinical trials with Type 1 dyslipidaemia is very limited and no studies were designed to especially investigate the effect of Omacor in these patients. Type HI dyslipidaemic patients are homozygotes for ApoE, and genotyping of patients was only performed in one study (K85-95011). More Type III dyslipidemic patients may have been therefore enrolled in clinical studies without being verified as such.
There is no reason to believe that Type HI dyslipidaemic patients do not respond to Omacor.
One of the pivotal clinical trials in patients with type IV and V (K85-95009 study) demonstrated a mean LDL-C increase of 42.6% with Omacor 4 g day. 67% of the patients in the study experienced increases in LDL-C, and the increases observed were in the range of 6%-110%. However, mean LDL-C concentrations at the end of the study were still only equal to 2.69 mmol/L (104 mg/dL). For the majority of these patients (40 of 42 with no history of coronary disease) this is still below their target LDL-C levels. Only equal to 2.69 mmol/L (104 mg/dL).
In clinical trials on patients with Type Hb dyslipidaemia mean LDL-C is unchanged or slightly increased (maximum 8.6%) with Omacor treatment. In studies with concomitant treatment of Omacor and a statin no significant increase in LDL-C has been observed with Omacor.
The cholesterol enrichment of LDL particles appears to happen in conjunction with a marked reduction in VLDL-C.
Studies also demonstrate a shift from small, dense LDL particles to larger, more buoyant LDL particles, indicating a shift towards less atherogenic lipoprotein particles.
Consistent with the overall population (see Table 5 hereafter), subjects in each baseline triglycerides level category in the Omacor 4 g treatment group had significantly larger mean absolute and relative changes in triglycerides levels compared with those in the placebo treatment group.
For the subjects who received Omacor 4 g per day, those with higher baseline levels (TG 500-749 mg/dL and 3750 mg/dL [5.65-8.46 mmol/L, and 8.47 mmol/L]) had greater reductions in triglycerides levels, and therefore were more likely to exhibit a better response to Omacor. (See Table 5).

A number of studies have been conducted to evaluate the effect of concomitant use of Omacor with widely used statins (simvastatin, atorvastatin). The studies have been carried out in patients with elevated serum triglycerides receiving statin therapy. The results of the studies demonstrate that the combined treatment increases the efficacy in lowering triglycerides. In these studies little or no effect on LDL-C has been observed and no significant safety issues have been raised.
Pharmacokinetics: The hydrolysis of omega-3 ethyl esters by esterases in the intestine is complete and rapid. After absorption, OFA are metabolised by multiple pathways that are not highly predictable. Animal pharmacokinetic studies have shown that there is no systemic exposure of the ethyl esters. Due to this complicated process, it is not possible to conduct standard bioavailability studies, and consequently, to measure meaningful values for Cmax, Tmax, AUC, etc. for Omacor.
The levels of EPA and DHA do increase on ingestion of Omacor, although in a less than dose proportional manner.
The absorption of Omacor has been determined by measuring the increase of EPA and DHA in plasma serum phospholipids after dosing. Significant, dose-dependent increases in serum phospholipids EPA content were seen, while increase in DHA incorporation were less marked and not dose dependent. Uptake of EPA and DHA into plasma/serum phospholipids in subjects treated with Omacor was also independent of gender, age, and hypertensive status. Concomitant ingestion of another unsaturated fatty acid, olive oil, did not affect absorption of omega-3 fatty acids from Omacor.
During and after absorption there are three main pathways for the metabolism of the omega-3 fatty acids: The fatty acids are first transported to the liver where they are incorporated into various categories of lipoproteins and then channeled to the peripheral lipids stores.
The cell membrane phospholipids are replaced by lipoprotein phospholipids and the fatty acids can then act as precursors for various eicosanoids.
The majority is oxidized to meet energy requirements.
The concentration of omega-3 fatty acids, EPA and DHA, in the plasma phospholipids corresponds to the EPA and DHA incorporated into the cell membranes.
MedsGo Class
Features
Dosage
460 mg / 380 mg
Ingredients
- DHA
- EPA
Packaging
SoftGel Capsule 1's
Generic Name
Eicossapentaenoic Acid (EPA) Ethyl Ester / Docosahexaenoic Acid (DHA) Ethyl ester
Registration Number
DR-XY25262
Classification
Prescription Drug (RX)
Product Questions
Questions

