Indications / Uses
Semaglutide is used to help adults with obesity, or adults with overweight who also have weight-related medical problems, to effectively lose excess body weight and maintain the weight loss. To reduce the risk of major cardiovascular events such as death, heart attack, or stroke in adults with known heart disease and with either obesity or overweight.
To manage diabetes in adults by regulating blood sugar levels, which can lead to improved overall health.
Expected Results
Effectiveness: Semaglutide mimics the hormone GLP-1, enhancing insulin secretion, reducing appetite, and slowing gastric emptying, leading to better glucose control and weight loss.
Onset: Improvements in blood sugar control may be observed within a few days, with weight loss effects noticeable within weeks to months of consistent use.
Clinical Evidence: Clinical trials have shown Semaglutide to significantly reduce HbA1c levels and promote weight loss in adults, supporting its use in diabetes and weight management.
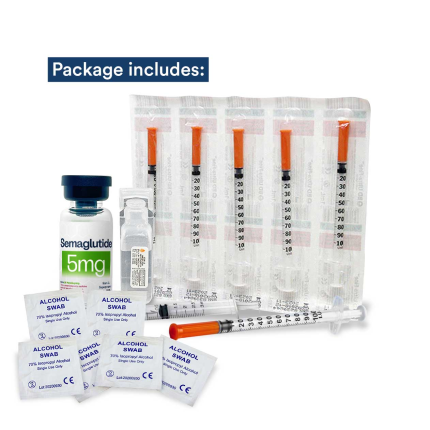
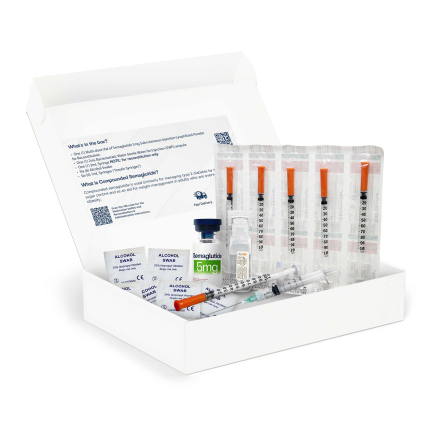
What's in the Box?

Your package should include:
- One (1) Multi-dose Vial of Semaglutide 5mg Subcutaneous Injection - Lyophilized Powder for Reconstitution
- One (1) 5mL Non-bacteriostatic Sterile Water for Injection (SWFI) ampule
- One (1) 3mL Syringe (for reconstitution only)
- Six (6) Alcohol Swabs
- Six (6) 1mL Syringes (“Insulin Syringes”)
Dosage / Direction
Administer as directed by your healthcare provider. The medication is typically given as a subcutaneous injection in the abdomen, thigh, or upper arm. Rotate injection sites with each dose to avoid irritation.
Dosing: Dosing should be directed by your doctor. The standard dosing is provided below:
- 0.25mg (0.1mL) each week for weeks 1 to 4
- 0.50mg (0.2mL) each week for weeks 5 to 8
- 1.00mg (0.4mL) each week for weeks 9 to 12
- 2.00mg (0.8mL) each week for weeks 13 and onward
Missed Dose: If a dose is missed, administer a missed dose as soon as possible within 5 days. If more than 5 days have passed, skip the missed dose and resume your regular schedule.
How to reconstitute Semaglutide?
- Clean your hands thoroughly and set up all components on a clean, organized surface.
- Wipe down any surfaces to eliminate potential contamination and prepare a designated sharps container for used needles.
- Examine all components, including multi-dose vials and syringes, to ensure they are intact, of good quality, and free of defects.
- Carefully twist open the cap of the 5mL non-bacteriostatic SWFI ampule and set it aside in an upright position.
- Remove the cap from the multi-dose vial to expose the rubber top and set it upright.
- Use an alcohol swab to wipe and disinfect the rubber top of the multi-dose vial and place the used swab on its original packaging for disposal.
- Using the 3mL syringe, draw 2mL of the non-bacteriostatic SWFI from the ampule.
- With one hand, hold the filled syringe steady, and with the other hand, insert the needle into the rubber top of the multi-dose vial.
- Slowly push the plunger to introduce the non-bacteriostatic SWFI into the vial, then mix the vial until a clear solution is achieved, removing the syringe once mixing is complete.
- Dispose of the used needle immediately in the designated sharps container.
Avoiding Needle-Stick Injury
- Use a one-handed technique when recapping used needles.
- Keep your workspace clutter-free to prevent accidents.
- Remove any potential sources of distraction or disturbance.
- You may use gloves and protective gear when handling needles.
- Report any needle-stick incidents immediately to a healthcare provider.
Administration Instructions
- Clean your hands and prepare a clean, organized surface with the reconstituted multi-dose vial, a 1mL syringe, and an alcohol swab.
- Wipe down surfaces to eliminate contamination and prepare a designated sharps container.
- Inspect all components to ensure they are intact and free of dirt or defects.
- Shake the reconstituted multi-dose vial to ensure the solution is uniform.
- Use an alcohol swab to disinfect the rubber top of the vial and dispose of the swab properly.
- While the syringe is empty, insert the syringe into the vial then draw the prescribed dose.
- Set the vial aside after filling the syringe, ensuring you measure accurately (i.e., use the top part of the plunger seal to measure the appropriate volume).
- For injection, pinch the skin and insert the needle at a 45º angle into the abdomen, thigh, or upper arm, rotating sites to prevent irritation.
- Inject the medication slowly and leave the needle in for 5 seconds after injecting.
- Remove and dispose of the needle in the sharps container.
RECONSTITUTION DEMO VIDEO
Side-effects
Common Semaglutide side effects include nausea, vomiting, diarrhea, abdominal pain, decreased appetite, and constipation.
Warnings:
- Thyroid Tumors: Report symptoms of thyroid tumors.
- Pancreatitis, Renal Failure: Report pancreatitis or renal failure symptoms; Maintain adequate hydration.
- Vision Changes: Report any vision changes.
- Gallbladder Disease: Report symptoms of gallbladder disease.
- Hypoglycemia: Monitor for symptoms of hypoglycemia and report any difficulties with glycemic control.
- Mental Health Changes: Report worsening depression, suicidal ideation, or unusual changes in behavior.
- Cardiac Symptoms: Report any increase in heart rate or heart palpitations while resting.
Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in Description.
Is it safe to take it with other drugs?
Semaglutide can slow down the emptying of your stomach, which may impact how quickly other medications you take by mouth get absorbed into your body.
Here's a breakdown of how Semaglutide interacts with some common medications:
- Paracetamol: Semaglutide may slightly delay the absorption of paracetamol, but the overall amount absorbed remains the same. No dosage adjustment is needed for paracetamol when taken with Semaglutide.
- Birth control pills: Semaglutide shouldn't affect the effectiveness of your birth control pills. Studies haven't shown any significant changes in hormone levels when taking both medications together.
- Atorvastatin (cholesterol medication): Semaglutide may slightly delay the absorption of atorvastatin, but the overall amount absorbed remains sufficient.
- Digoxin (heart medication): Semaglutide doesn't affect the absorption or peak levels of digoxin in your body.
- Metformin (diabetes medication): Semaglutide doesn't affect the absorption or peak levels of metformin in your body.
- Warfarin (blood thinner): Semaglutide doesn't directly affect warfarin, but it's recommended to monitor your blood clotting levels (INR) more frequently when starting Semaglutide while taking warfarin or similar blood thinners.
How should I store it?
- Unreconstituted Lyophilized Powder is stable at room temperatures of 18ºC to 25ºC (64°F to 77°F) and should not be refrigerated.
- Reconstituted Lyophilized Powder should be stored in a refrigerator at 2°C to 8°C (36°F to 46°F). Do not freeze.
- Once in use, Reconstituted Lyophilized Powder must be stored in a refrigerator at 2°C to 8°C (36°F to 46°F).
Expiry and beyond use dates
- Expiry as written on the vial (1-2 years)
- Beyond-Use Date: Reconstituted Lyophilized Powder should only be used 30 days from reconstitution.
___________
This is a prescription-only medicine and a compounded medication. The law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed.
NOTE: Please note that the following is a generic description of compounded semaglutide medicine. We do not maintain any pre-compounded stock of this product. The compounded medicine is prepared only after an order is placed and upon receipt of a valid prescription or following a teleconsultation with a licensed doctor. This information is provided for general informational purposes only and does not constitute medical advice, a recommendation for treatment, or an endorsement of any specific therapy. The preparation of the compounded medicine is subject to applicable local regulations and standards.