Indications/Uses
Endocrine disorders: Primary and secondary adrenal insufficiency, Congenital adrenal hyperplasia.
Rheumatic disorders: Rheumatoid arthritis, Juvenile chronic arthritis, Ankylosing spondylitis.
Collagen diseases/arteritis: Systemic lupus erythematosus, Systemic dermatomyositis (polymyositis), Rheumatic fever with severe carditis, Giant cell arteritis/polymyalgia rheumatica.
Dermatological diseases: Pemphigus vulgaris.
Allergic states: Severe seasonal and perennial allergic rhinitis, Drug hypersensitivity reactions, Serum sickness, Allergic contact dermatitis, Bronchial asthma.
Ophthalmic diseases: Anterior uveitis (iritis, iridocyclitis), Posterior uveitis, Optic neuritis.
Respiratory diseases: Pulmonary sarcoid, Fulminating or disseminated tuberculosis (with appropriate anti-tuberculous chemotherapy), Aspiration of gastric contents.
Haematological disorders: Idiopathic thrombocytopenic purpura, Haemolytic anaemia (autoimmune).
Neoplastic diseases: Leukaemia (acute and lymphatic), Malignant lymphoma.
Gastro-intestinal diseases: Ulcerative colitis, Crohn's disease.
Miscellaneous: Tuberculous meningitis (with appropriate anti-tuberculous chemotherapy), Transplantation.
Rheumatic disorders: Rheumatoid arthritis, Juvenile chronic arthritis, Ankylosing spondylitis.
Collagen diseases/arteritis: Systemic lupus erythematosus, Systemic dermatomyositis (polymyositis), Rheumatic fever with severe carditis, Giant cell arteritis/polymyalgia rheumatica.
Dermatological diseases: Pemphigus vulgaris.
Allergic states: Severe seasonal and perennial allergic rhinitis, Drug hypersensitivity reactions, Serum sickness, Allergic contact dermatitis, Bronchial asthma.
Ophthalmic diseases: Anterior uveitis (iritis, iridocyclitis), Posterior uveitis, Optic neuritis.
Respiratory diseases: Pulmonary sarcoid, Fulminating or disseminated tuberculosis (with appropriate anti-tuberculous chemotherapy), Aspiration of gastric contents.
Haematological disorders: Idiopathic thrombocytopenic purpura, Haemolytic anaemia (autoimmune).
Neoplastic diseases: Leukaemia (acute and lymphatic), Malignant lymphoma.
Gastro-intestinal diseases: Ulcerative colitis, Crohn's disease.
Miscellaneous: Tuberculous meningitis (with appropriate anti-tuberculous chemotherapy), Transplantation.
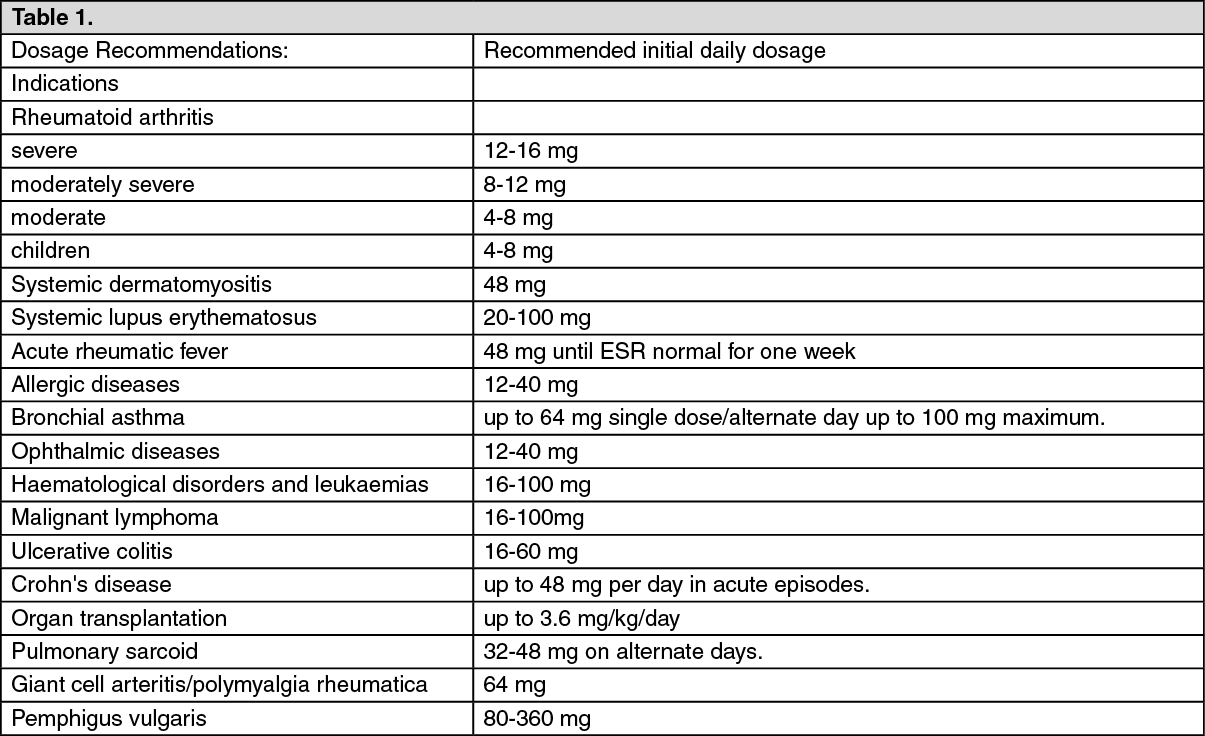
Dosage/Direction for Use
The dosage recommendations shown in the table as follows are suggested initial daily doses and are intended as guides. The average total daily dose recommended may be given either as a single dose or in divided doses (excepting in alternate day therapy when the minimum effective daily dose is doubled and given every other day at 8.00 am).
Undesirable effects may be minimised by using the lowest effective dose for the minimum period (see Precautions).
The initial suppressive dose level may vary depending on the condition being treated. This is continued until a satisfactory clinical response is obtained, a period usually of three to seven days in the case of rheumatic diseases (except for acute rheumatic carditis), allergic conditions affecting the skin or respiratory tract and ophthalmic diseases. If a satisfactory response is not obtained in seven days, re-evaluation of the case to confirm the original diagnosis should be made. As soon as a satisfactory clinical response is obtained, the daily dose should be reduced gradually, either to termination of treatment in the case of acute conditions (e.g. seasonal asthma, exfoliative dermatitis, acute ocular inflammations) or to the minimal effective maintenance dose level in the case of chronic conditions (e.g. rheumatoid arthritis, systemic lupus erythematosus, bronchial asthma, atopic dermatitis). In chronic conditions, and in rheumatoid arthritis especially, it is important that the reduction in dosage from initial to maintenance dose levels be accomplished as clinically appropriate. Decrements of not more than 2 mg at intervals of 7 10 days are suggested. In rheumatoid arthritis, maintenance steroid therapy should be at the lowest possible level.
In alternate-day therapy, the minimum daily corticoid requirement is doubled and administered as a single dose every other day at 8.00 am. Dosage requirements depend on the condition being treated and response of the patient.
Elderly patients: Treatment of elderly patients, particularly if long-term, should be planned bearing in mind the more serious consequences of the common side-effects of corticosteroids in old age, particularly osteoporosis, diabetes, hypertension, susceptibility to infection and thinning of skin (see Precautions).
Children: In general, dosage for children should be based upon clinical response and is at the discretion of the physician. Treatment should be limited to the minimum dosage for the shortest period of time. If possible, treatment should be administered as a single dose on alternate days (see Precautions). (See Table 1.)
Undesirable effects may be minimised by using the lowest effective dose for the minimum period (see Precautions).
The initial suppressive dose level may vary depending on the condition being treated. This is continued until a satisfactory clinical response is obtained, a period usually of three to seven days in the case of rheumatic diseases (except for acute rheumatic carditis), allergic conditions affecting the skin or respiratory tract and ophthalmic diseases. If a satisfactory response is not obtained in seven days, re-evaluation of the case to confirm the original diagnosis should be made. As soon as a satisfactory clinical response is obtained, the daily dose should be reduced gradually, either to termination of treatment in the case of acute conditions (e.g. seasonal asthma, exfoliative dermatitis, acute ocular inflammations) or to the minimal effective maintenance dose level in the case of chronic conditions (e.g. rheumatoid arthritis, systemic lupus erythematosus, bronchial asthma, atopic dermatitis). In chronic conditions, and in rheumatoid arthritis especially, it is important that the reduction in dosage from initial to maintenance dose levels be accomplished as clinically appropriate. Decrements of not more than 2 mg at intervals of 7 10 days are suggested. In rheumatoid arthritis, maintenance steroid therapy should be at the lowest possible level.
In alternate-day therapy, the minimum daily corticoid requirement is doubled and administered as a single dose every other day at 8.00 am. Dosage requirements depend on the condition being treated and response of the patient.
Elderly patients: Treatment of elderly patients, particularly if long-term, should be planned bearing in mind the more serious consequences of the common side-effects of corticosteroids in old age, particularly osteoporosis, diabetes, hypertension, susceptibility to infection and thinning of skin (see Precautions).
Children: In general, dosage for children should be based upon clinical response and is at the discretion of the physician. Treatment should be limited to the minimum dosage for the shortest period of time. If possible, treatment should be administered as a single dose on alternate days (see Precautions). (See Table 1.)
Overdosage
Administration of methylprednisolone should not be discontinued abruptly but tailed off over a period of time. Appropriate action should be taken to alleviate the symptoms produced by any side-effect that may become apparent. It may be necessary to support the patient with corticosteroids during any further period of trauma occurring within two years of overdosage.
There is no clinical syndrome of acute overdose with methylprednisolone. Reports of acute toxicity and/or death following overdosage of glucocorticoids are rare. In the event of overdosage, no specific antidote is available; treatment is supportive and symptomatic. Methylprednisolone is haemodialysable.
There is no clinical syndrome of acute overdose with methylprednisolone. Reports of acute toxicity and/or death following overdosage of glucocorticoids are rare. In the event of overdosage, no specific antidote is available; treatment is supportive and symptomatic. Methylprednisolone is haemodialysable.
Administration
Should be taken with food.
Contraindications
Methylprednisolone tablets are contraindicated in patients who have: systemic fungal infections; systemic infections unless specific anti-infective therapy is employed; hypersensitivity to the active substance or to any of the excipients.
Special Precautions
Immunosuppressant Effects/Increased Susceptibility to Infections: Corticosteroids may increase susceptibility to infection, may mask some signs of infection, and new infections may appear during their use. Suppression of the inflammatory response and immune function increases the susceptibility to fungal, viral and bacterial infections and their severity. The clinical presentation may often be atypical and may reach an advanced stage before being recognised.
Persons who are on drugs which suppress the immune system are more susceptible to infections than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in non-immune children or adults on corticosteroids.
Chickenpox is of serious concern since this normally minor illness may be fatal in immunosuppressed patients. Patients (or parents of children) without a definite history of chickenpox should be advised to avoid close personal contact with chickenpox or herpes zoster and if exposed they should seek urgent medical attention. Passive immunization with varicella/zoster immunoglobulin (VZIG) is needed by exposed non-immune patients who are receiving systemic corticosteroids or who have used them within the previous 3 months; this should be given within 10 days of exposure to chickenpox. If a diagnosis of chickenpox is confirmed, the illness warrants specialist care and urgent treatment. Corticosteroids should not be stopped and the dose may need to be increased.
Exposure to measles should be avoided. Medical advice must be sought immediately if exposure occurs. Prophylaxis with normal intramuscular immunoglobulin may be needed.
Similarly corticosteroids should be used with great care in patients with known or suspected parasitic infections such as Strongyloides (threadworm) infestation, which may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia. The role of corticosteroids in septic shock has been controversial, with early studies reporting both beneficial and detrimental effects. More recently, supplemental corticosteroids have been suggested to be beneficial in patients with established septic shock who exhibit adrenal insufficiency. However, their routine use in septic shock is not recommended. A systematic review of short-course high-dose corticosteroids did not support their use. However, meta-analyses, and a review have suggested that longer courses (5-11 days) of low-dose corticosteroids might reduce mortality.
Live vaccines should not be given to individuals with impaired immune responsiveness. The antibody response to other vaccines may be diminished.
The use of corticosteroids in active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate antituberculous regimen. If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
Kaposi's sarcoma has been reported to occur in patients receiving corticosteroid therapy. Discontinuation of corticosteroids may result in clinical remission.
Blood and Lymphatic System: Aspirin and nonsteroidal anti-inflammatory agents should be used cautiously in conjunction with corticosteroids.
Endocrine Effects: Adrenal cortical atrophy develops during prolonged therapy and may persist for months after stopping treatment. In patients who have received more than physiological doses of systemic corticosteroids (approximately 6 mg methylprednisolone) for greater than 3 weeks, withdrawal should not be abrupt. How dose reduction should be carried out depends largely on whether the disease is likely to relapse as the dose of systemic corticosteroids is reduced. Clinical assessment of disease activity may be needed during withdrawal. If the disease is unlikely to relapse on withdrawal of systemic corticosteroids, but there is uncertainty about HPA suppression, the dose of systemic corticosteroid may be reduced rapidly to physiological doses. Once a daily dose of 6 mg methylprednisolone is reached, dose reduction should be slower to allow the HPA-axis to recover.
Abrupt withdrawal of systemic corticosteroid treatment, which has continued up to 3 weeks is appropriate if it considered that the disease is unlikely to relapse. Abrupt withdrawal of doses up to 32 mg daily of methylprednisolone for 3 weeks is unlikely to lead to clinically relevant HPA-axis suppression, in the majority of patients. In the following patient groups, gradual withdrawal of systemic corticosteroid therapy should be considered even after courses lasting 3 weeks or less: Patients who have had repeated courses of systemic corticosteroids, particularly if taken for greater than 3 weeks. When a short course has been prescribed within one year of cessation of long-term therapy (months or years).
Patients who may have reasons for adrenocortical insufficiency other than exogenous corticosteroid therapy. In addition, acute adrenal insufficiency leading to a fatal outcome may occur if glucocorticoids are withdrawn abruptly.
Patients receiving doses of systemic corticosteroid greater than 32 mg daily of methylprednisolone.
Patients repeatedly taking doses in the evening.
In drug-induced adrenocorticol insufficiency mineralocorticoid secretion may be impaired, therefore salt and/or a mineralocorticoid should be administered concurrently.
Glucocorticoids can produce or aggravate Cushing's syndrome, therefore glucocorticoids should be avoided in patients with Cushing's disease.
Particular care is required when considering the use of systemic corticosteroids in patients with hypothyroidism and frequent patient monitoring is necessary.
Metabolism and Nutrition Disorders: Corticosteroids, including methylprednisolone, can increase blood glucose, worsen pre-existing diabetes, and predispose those on long-term corticosteroid therapy to diabetes mellitus.
Particular care is required when considering the use of systemic corticosteroids in patients with Diabetes mellitus (or a family history of diabetes) and frequent patient monitoring is necessary.
Psychiatric Effects: Patients and/or carers should be warned that potentially severe psychiatric adverse reactions may occur with systemic steroids. Symptoms typically emerge within a few days or weeks of starting treatment. Risks may be higher with high doses/systemic exposure, although dose levels do not allow prediction of the onset, type, severity or duration of reactions. Most reactions recover after either dose reduction or withdrawal, although specific treatment may be necessary. Patients/carers should be encouraged to seek medical advice if worrying psychological symptoms develop, especially if depressed mood or suicidal ideation is suspected. Patients/carers should be alert to possible psychiatric disturbances that may occur either during or immediately after dose tapering/withdrawal of systemic steroids, although such reactions have been reported infrequently.
Particular care is required when considering the use of systemic corticosteroids in patients with existing or previous history of severe affective disorders in themselves or in their first degree relatives. These would include depressive or manic-depressive illness and previous steroid psychosis.
Nervous System Effects: Particular care is required when considering the use of systemic corticosteroids in patients with seizure disorders and myasthenia gravis and frequent patient monitoring is necessary.
Ocular Effects: Particular care is required when considering the use of systemic corticosteroids in patients with glaucoma (or a family history of glaucoma) and ocular herpes simplex as there is a fear of corneal perforation, and frequent patient monitoring is necessary.
Prolonged use of corticosteroids may produce posterior subcapsular cataracts and nuclear cataracts (particularly in children), exophthalmos or increased intraocular pressure, which may result in glaucoma with possible damage to the optic nerves.
Secondary fungal and viral infections of the eye may also be enhanced in patients receiving glucocorticoids.
Cardiac events: Systemic corticosteroids should be used with caution, and only if strictly necessary, in cases of congestive heart failure. Particular care is required when considering the use of systemic corticosteroids in patients with recent myocardial infarction (myocardial rupture has been reported) and frequent patient monitoring is necessary.
Care should be taken for patients receiving cardioactive drugs such as digoxin because of steroid induced electrolyte disturbance/potassium loss.
Vascular Effects: Particular care is required when considering the use of systemic corticosteroids in patients with the following conditions and frequent patient monitoring is necessary: Hypertension; Predisposition to thrombophlebitis.
Gastrointestinal Effects: Particular care is required when considering the use of systemic corticosteroids in patients with the following conditions and frequent patient monitoring is necessary: Peptic ulceration; Fresh intestinal anastomoses; Abscess or other pyogenic infections; Ulcerative colitis; Diverticulitis.
Hepatobiliary Effects: There is an enhanced effect of corticosteroids on patients with cirrhosis.
Particular care is required when considering the use of systemic corticosteroids in patients with liver failure or cirrhosis and frequent patient monitoring is necessary.
Musculoskeletal Effects: An acute myopathy has been reported with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (eg, myasthenia gravis), or in patients receiving concomitant therapy with anticholinergics, such as neuromuscular blocking drugs (eg, pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevations of creatine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
Particular care is required when considering the use of systemic corticosteroids in patients with osteoporosis (post-menopausal females are particularly at risk) and frequent patient monitoring is necessary.
Renal and Urinary: Particular care is required when considering the use of systemic corticosteroids in patients with renal insufficiency and frequent patient monitoring is necessary.
Injury, poisoning and procedural complications: High doses of systemic corticosteroids should not be used for the treatment of traumatic brain injury.
Other: Undesirable effects may be minimized by using the lowest effective dose for the minimum period, and by administering the daily requirement as a single morning dose or whenever possible as a single morning dose on alternative days. Frequent patient review is required to appropriately titrate the dose against disease activity.
Patients should carry 'Steroid Treatment' cards which give clear guidance on the precautions to be taken to minimize risk and which provide details of prescriber, drug, dosage and the duration of treatment.
Ingredient warning: This medicine contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
This medicine contains sucrose. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine.
Use in Children: Corticosteroids cause growth retardation in infancy, childhood and adolescence. Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed. Treatment should be limited to the minimum dosage for the shortest possible time. In order to minimize suppression of the hypothalamo-pituitary-adrenal axis and growth retardation, treatment should be administered where possible as a single dose on alternate days.
Use in Elderly: The common adverse effects of systemic corticosteroids may be associated with more serious consequences in old age, especially osteoporosis, hypertension, hypokalaemia, diabetes, susceptibility to infection and thinning of the skin. Close clinical supervision is required to avoid life-threatening reactions.
Persons who are on drugs which suppress the immune system are more susceptible to infections than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in non-immune children or adults on corticosteroids.
Chickenpox is of serious concern since this normally minor illness may be fatal in immunosuppressed patients. Patients (or parents of children) without a definite history of chickenpox should be advised to avoid close personal contact with chickenpox or herpes zoster and if exposed they should seek urgent medical attention. Passive immunization with varicella/zoster immunoglobulin (VZIG) is needed by exposed non-immune patients who are receiving systemic corticosteroids or who have used them within the previous 3 months; this should be given within 10 days of exposure to chickenpox. If a diagnosis of chickenpox is confirmed, the illness warrants specialist care and urgent treatment. Corticosteroids should not be stopped and the dose may need to be increased.
Exposure to measles should be avoided. Medical advice must be sought immediately if exposure occurs. Prophylaxis with normal intramuscular immunoglobulin may be needed.
Similarly corticosteroids should be used with great care in patients with known or suspected parasitic infections such as Strongyloides (threadworm) infestation, which may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia. The role of corticosteroids in septic shock has been controversial, with early studies reporting both beneficial and detrimental effects. More recently, supplemental corticosteroids have been suggested to be beneficial in patients with established septic shock who exhibit adrenal insufficiency. However, their routine use in septic shock is not recommended. A systematic review of short-course high-dose corticosteroids did not support their use. However, meta-analyses, and a review have suggested that longer courses (5-11 days) of low-dose corticosteroids might reduce mortality.
Live vaccines should not be given to individuals with impaired immune responsiveness. The antibody response to other vaccines may be diminished.
The use of corticosteroids in active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate antituberculous regimen. If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
Kaposi's sarcoma has been reported to occur in patients receiving corticosteroid therapy. Discontinuation of corticosteroids may result in clinical remission.
Blood and Lymphatic System: Aspirin and nonsteroidal anti-inflammatory agents should be used cautiously in conjunction with corticosteroids.
Endocrine Effects: Adrenal cortical atrophy develops during prolonged therapy and may persist for months after stopping treatment. In patients who have received more than physiological doses of systemic corticosteroids (approximately 6 mg methylprednisolone) for greater than 3 weeks, withdrawal should not be abrupt. How dose reduction should be carried out depends largely on whether the disease is likely to relapse as the dose of systemic corticosteroids is reduced. Clinical assessment of disease activity may be needed during withdrawal. If the disease is unlikely to relapse on withdrawal of systemic corticosteroids, but there is uncertainty about HPA suppression, the dose of systemic corticosteroid may be reduced rapidly to physiological doses. Once a daily dose of 6 mg methylprednisolone is reached, dose reduction should be slower to allow the HPA-axis to recover.
Abrupt withdrawal of systemic corticosteroid treatment, which has continued up to 3 weeks is appropriate if it considered that the disease is unlikely to relapse. Abrupt withdrawal of doses up to 32 mg daily of methylprednisolone for 3 weeks is unlikely to lead to clinically relevant HPA-axis suppression, in the majority of patients. In the following patient groups, gradual withdrawal of systemic corticosteroid therapy should be considered even after courses lasting 3 weeks or less: Patients who have had repeated courses of systemic corticosteroids, particularly if taken for greater than 3 weeks. When a short course has been prescribed within one year of cessation of long-term therapy (months or years).
Patients who may have reasons for adrenocortical insufficiency other than exogenous corticosteroid therapy. In addition, acute adrenal insufficiency leading to a fatal outcome may occur if glucocorticoids are withdrawn abruptly.
Patients receiving doses of systemic corticosteroid greater than 32 mg daily of methylprednisolone.
Patients repeatedly taking doses in the evening.
In drug-induced adrenocorticol insufficiency mineralocorticoid secretion may be impaired, therefore salt and/or a mineralocorticoid should be administered concurrently.
Glucocorticoids can produce or aggravate Cushing's syndrome, therefore glucocorticoids should be avoided in patients with Cushing's disease.
Particular care is required when considering the use of systemic corticosteroids in patients with hypothyroidism and frequent patient monitoring is necessary.
Metabolism and Nutrition Disorders: Corticosteroids, including methylprednisolone, can increase blood glucose, worsen pre-existing diabetes, and predispose those on long-term corticosteroid therapy to diabetes mellitus.
Particular care is required when considering the use of systemic corticosteroids in patients with Diabetes mellitus (or a family history of diabetes) and frequent patient monitoring is necessary.
Psychiatric Effects: Patients and/or carers should be warned that potentially severe psychiatric adverse reactions may occur with systemic steroids. Symptoms typically emerge within a few days or weeks of starting treatment. Risks may be higher with high doses/systemic exposure, although dose levels do not allow prediction of the onset, type, severity or duration of reactions. Most reactions recover after either dose reduction or withdrawal, although specific treatment may be necessary. Patients/carers should be encouraged to seek medical advice if worrying psychological symptoms develop, especially if depressed mood or suicidal ideation is suspected. Patients/carers should be alert to possible psychiatric disturbances that may occur either during or immediately after dose tapering/withdrawal of systemic steroids, although such reactions have been reported infrequently.
Particular care is required when considering the use of systemic corticosteroids in patients with existing or previous history of severe affective disorders in themselves or in their first degree relatives. These would include depressive or manic-depressive illness and previous steroid psychosis.
Nervous System Effects: Particular care is required when considering the use of systemic corticosteroids in patients with seizure disorders and myasthenia gravis and frequent patient monitoring is necessary.
Ocular Effects: Particular care is required when considering the use of systemic corticosteroids in patients with glaucoma (or a family history of glaucoma) and ocular herpes simplex as there is a fear of corneal perforation, and frequent patient monitoring is necessary.
Prolonged use of corticosteroids may produce posterior subcapsular cataracts and nuclear cataracts (particularly in children), exophthalmos or increased intraocular pressure, which may result in glaucoma with possible damage to the optic nerves.
Secondary fungal and viral infections of the eye may also be enhanced in patients receiving glucocorticoids.
Cardiac events: Systemic corticosteroids should be used with caution, and only if strictly necessary, in cases of congestive heart failure. Particular care is required when considering the use of systemic corticosteroids in patients with recent myocardial infarction (myocardial rupture has been reported) and frequent patient monitoring is necessary.
Care should be taken for patients receiving cardioactive drugs such as digoxin because of steroid induced electrolyte disturbance/potassium loss.
Vascular Effects: Particular care is required when considering the use of systemic corticosteroids in patients with the following conditions and frequent patient monitoring is necessary: Hypertension; Predisposition to thrombophlebitis.
Gastrointestinal Effects: Particular care is required when considering the use of systemic corticosteroids in patients with the following conditions and frequent patient monitoring is necessary: Peptic ulceration; Fresh intestinal anastomoses; Abscess or other pyogenic infections; Ulcerative colitis; Diverticulitis.
Hepatobiliary Effects: There is an enhanced effect of corticosteroids on patients with cirrhosis.
Particular care is required when considering the use of systemic corticosteroids in patients with liver failure or cirrhosis and frequent patient monitoring is necessary.
Musculoskeletal Effects: An acute myopathy has been reported with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (eg, myasthenia gravis), or in patients receiving concomitant therapy with anticholinergics, such as neuromuscular blocking drugs (eg, pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevations of creatine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
Particular care is required when considering the use of systemic corticosteroids in patients with osteoporosis (post-menopausal females are particularly at risk) and frequent patient monitoring is necessary.
Renal and Urinary: Particular care is required when considering the use of systemic corticosteroids in patients with renal insufficiency and frequent patient monitoring is necessary.
Injury, poisoning and procedural complications: High doses of systemic corticosteroids should not be used for the treatment of traumatic brain injury.
Other: Undesirable effects may be minimized by using the lowest effective dose for the minimum period, and by administering the daily requirement as a single morning dose or whenever possible as a single morning dose on alternative days. Frequent patient review is required to appropriately titrate the dose against disease activity.
Patients should carry 'Steroid Treatment' cards which give clear guidance on the precautions to be taken to minimize risk and which provide details of prescriber, drug, dosage and the duration of treatment.
Ingredient warning: This medicine contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
This medicine contains sucrose. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine.
Use in Children: Corticosteroids cause growth retardation in infancy, childhood and adolescence. Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed. Treatment should be limited to the minimum dosage for the shortest possible time. In order to minimize suppression of the hypothalamo-pituitary-adrenal axis and growth retardation, treatment should be administered where possible as a single dose on alternate days.
Use in Elderly: The common adverse effects of systemic corticosteroids may be associated with more serious consequences in old age, especially osteoporosis, hypertension, hypokalaemia, diabetes, susceptibility to infection and thinning of the skin. Close clinical supervision is required to avoid life-threatening reactions.
Adverse Reactions
Fluid and Electrolyte Disturbances: Sodium retention.
Congestive heart failure in susceptible patients: Hypertension, Fluid retention, Potassium loss, Hypokalemic alkalosis.
Musculoskeletal: Muscle weakness, Loss of muscle mass, Steroid myopathy, Osteoporosis, Vertebral compression fractures, Aseptic necrosis of femoral and humeral heads, Pathologic fracture of long bones.
Gastrointestinal: Peptic ulcer with possible perforation and hemorrhage.
Pancreatitis: Abdominal distention, Ulcerative esophagitis.
Dermatologic: Impaired wound healing, Petechiae and ecchymoses. May suppress reactions to skin tests. Thin fragile skin, Facial erythema, Increased sweating.
Neurological: Increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually after treatment, Convulsions, Vertigo, Headache.
Endocrine: Development of Cushingoid state; Suppression of growth in children; Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness; Menstrual irregularities; Decreased carbohydrate tolerance; Manifestations of latent diabetes mellitus; Increased requirements for insulin or oral hypoglycemic agents in diabetics.
Ophthalmic: Posterior subcapsular cataracts, Increased intraocular pressure, Glaucoma, Exophthalmos.
Metabolic: Negative nitrogen balance due to protein catabolism.
The following additional reactions have been reported following oral as well as parenteral therapy: Urticaria and other allergic, anaphylactic or hypersensitivity reactions.
Congestive heart failure in susceptible patients: Hypertension, Fluid retention, Potassium loss, Hypokalemic alkalosis.
Musculoskeletal: Muscle weakness, Loss of muscle mass, Steroid myopathy, Osteoporosis, Vertebral compression fractures, Aseptic necrosis of femoral and humeral heads, Pathologic fracture of long bones.
Gastrointestinal: Peptic ulcer with possible perforation and hemorrhage.
Pancreatitis: Abdominal distention, Ulcerative esophagitis.
Dermatologic: Impaired wound healing, Petechiae and ecchymoses. May suppress reactions to skin tests. Thin fragile skin, Facial erythema, Increased sweating.
Neurological: Increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually after treatment, Convulsions, Vertigo, Headache.
Endocrine: Development of Cushingoid state; Suppression of growth in children; Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness; Menstrual irregularities; Decreased carbohydrate tolerance; Manifestations of latent diabetes mellitus; Increased requirements for insulin or oral hypoglycemic agents in diabetics.
Ophthalmic: Posterior subcapsular cataracts, Increased intraocular pressure, Glaucoma, Exophthalmos.
Metabolic: Negative nitrogen balance due to protein catabolism.
The following additional reactions have been reported following oral as well as parenteral therapy: Urticaria and other allergic, anaphylactic or hypersensitivity reactions.
Drug Interactions
Methylprednisolone is a cytochrome P450 enzyme (CYP) substrate and is mainly metabolized by the CYP3A4 enzyme. CYP3A4 is the dominant enzyme of the most abundant CYP subfamily in the liver of adult humans. It catalyzes 6β-hydroxylation of steroids, the essential Phase I metabolic step for both endogenous and synthetic corticosteroids. Many other compounds are also substrates of CYP3A4, some of which (as well as other drugs) have been shown to alter glucocorticoid metabolism by induction (up regulation) or inhibition of the CYP3A4 enzyme. (See Table 2.)
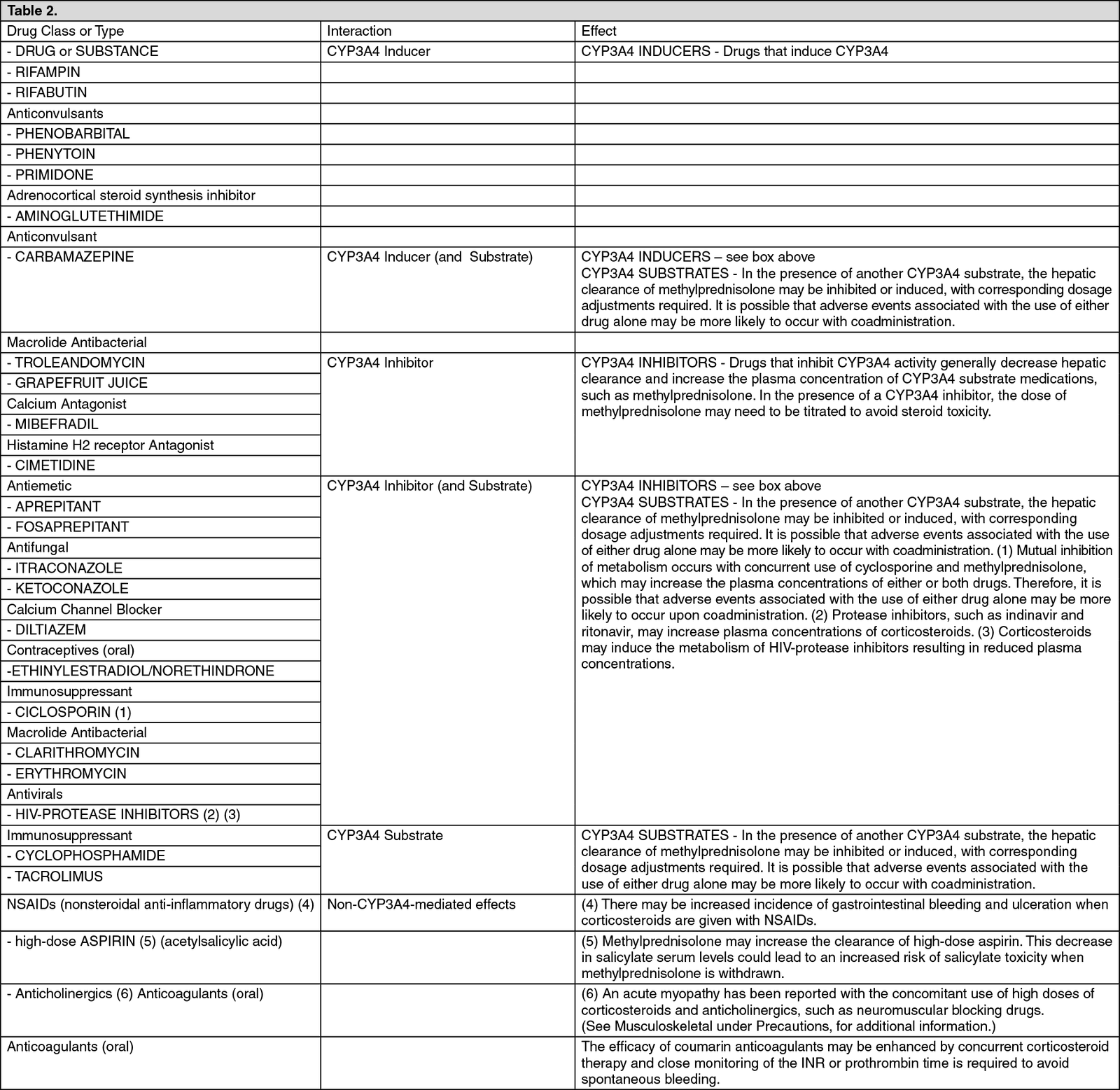
Steroids may reduce the effects of anticholinesterases in myasthenia gravis. The desired effects of hypoglycaemic agents (including
insulin), anti-hypertensives and diuretics are antagonised by corticosteroids, and the hypokalaemic effects of acetazolamide, loop diuretics, thiazide diuretics and carbenoxolone are enhanced.
Storage
Store at temperatures not exceeding 30°C. Protect from light.
Shelf Life: 3 Years from the date of manufacturing.
Shelf Life: 3 Years from the date of manufacturing.
Action
Pharmacological Classification: Corticosteroid (Glucocorticoid).
Pharmacology: Unbound glucocorticoids cross cell membranes and bind with high affinity to specific cytoplasmic receptors, modifying transcription and protein synthesis. By this mechanism, glucocorticoids can inhibit leukocyte infiltration at the site of inflammation, interfere with mediators of inflammatory response, and suppress humoral immune responses. The anti-inflammatory actions of corticosteroids are thought to involve phospholipase A2 inhibitory proteins, lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes.
Pharmacology: Unbound glucocorticoids cross cell membranes and bind with high affinity to specific cytoplasmic receptors, modifying transcription and protein synthesis. By this mechanism, glucocorticoids can inhibit leukocyte infiltration at the site of inflammation, interfere with mediators of inflammatory response, and suppress humoral immune responses. The anti-inflammatory actions of corticosteroids are thought to involve phospholipase A2 inhibitory proteins, lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes.
MedsGo Class
Corticosteroid Hormones
Features
Dosage
16 mg
Ingredients
- Methylprednisolone
Packaging
Tablet 1's
Generic Name
Methylprednisolone
Registration Number
DR-XY44853
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions


