Indications/Uses
Adults: Olanzapine is indicated for the acute and maintenance treatment of schizophrenia and other psychoses where positive symptoms (e.g., delusions, hallucinations, disordered thinking, hostility, and suspiciousness) and/or negative symptoms (e.g., flattened affect, emotional and social withdrawal, poverty of speech) are prominent. Olanzapine also alleviates the secondary affective symptoms commonly associated with schizophrenia and related disorders.
Olanzapine is effective in maintaining the clinical improvement during continuing therapy in patients who have shown initial treatment response. Olanzapine as monotherapy or in combination with lithium or valproate is indicated for the treatment of acute manic or mixed episodes in bipolar disorder with or without psychotic features and with or without a rapid cycling course.
Olanzapine is indicated for the prevention of recurrence in patients with bipolar mania.
Adolescents: Olanzapine is indicated for the acute treatment of schizophrenia in adolescent patients (aged 13 to 17 years).
Olanzapine is indicated for the acute manic and mixed episodes associated with bipolar disorder in adolescent patients (aged 13 to 17 years).
Olanzapine is effective in maintaining the clinical improvement during continuing therapy in patients who have shown initial treatment response. Olanzapine as monotherapy or in combination with lithium or valproate is indicated for the treatment of acute manic or mixed episodes in bipolar disorder with or without psychotic features and with or without a rapid cycling course.
Olanzapine is indicated for the prevention of recurrence in patients with bipolar mania.
Adolescents: Olanzapine is indicated for the acute treatment of schizophrenia in adolescent patients (aged 13 to 17 years).
Olanzapine is indicated for the acute manic and mixed episodes associated with bipolar disorder in adolescent patients (aged 13 to 17 years).
Dosage/Direction for Use
Adults: Schizophrenia and Related Disorders: The recommended starting dose for olanzapine is 10 mg administered once a day.
Acute mania associated with Bipolar Disorder: The recommended starting dose for Olanzapine is 15 mg administered once a day as monotherapy or 10 mg administered once daily in combination therapy with lithium or valproate.
Preventing recurrence in bipolar disorder: The recommended starting dose is 10 mg/day. For patients who have been receiving olanzapine for treatment of manic episode, continue therapy for preventing recurrence at the same dose. If a new manic, mixed, or depressive episode occurs, olanzapine treatment should be continued (with dose optimization as needed), with supplementary therapy to treat mood symptoms, as clinically indicated.
During treatment for schizophrenia, manic episode and recurrence prevention in bipolar disorder, daily dosage may subsequently be adjusted on the basis of individual clinical status within the range 5-20 mg/day. An increase to a dose greater than the recommended starting dose is advised only after appropriate clinical reassessment and should generally occur at intervals of not less than 24 hours. Olanzapine can be given without regards for meals as absorption is not affected by food. Gradual tapering of the dose should be considered when discontinuing olanzapine.
A lower starting dose of 5 mg per day may be considered for geriatric patients or when other clinical factors warrant. A 5 mg starting dose also may be considered for patients with severe renal or moderate hepatic impairment.
A lower starting dose may be considered in patients who exhibit a combination of factors (female gender, geriatric age, non-smoking status) which may slow the metabolism of olanzapine. Olanzapine has not been studied in subjects under 13 years of age.
Adolescents: Schizophrenia and Acute Manic or Mixed Episodes Associated with Bipolar Disorder in Adolescents: The recommended starting dose for olanzapine is 2.5 or 5 mg administered once a day. It may be given without regard to meals as its absorption is not affected by food. The dosage range of olanzapine in adolescents is 2.5 to 20 mg per day. Daily dosage should be adjusted on the basis of clinical status. When dosage adjustments are necessary, dose increments/decrements of 2.5 or 5 mg are recommended.
Acute mania associated with Bipolar Disorder: The recommended starting dose for Olanzapine is 15 mg administered once a day as monotherapy or 10 mg administered once daily in combination therapy with lithium or valproate.
Preventing recurrence in bipolar disorder: The recommended starting dose is 10 mg/day. For patients who have been receiving olanzapine for treatment of manic episode, continue therapy for preventing recurrence at the same dose. If a new manic, mixed, or depressive episode occurs, olanzapine treatment should be continued (with dose optimization as needed), with supplementary therapy to treat mood symptoms, as clinically indicated.
During treatment for schizophrenia, manic episode and recurrence prevention in bipolar disorder, daily dosage may subsequently be adjusted on the basis of individual clinical status within the range 5-20 mg/day. An increase to a dose greater than the recommended starting dose is advised only after appropriate clinical reassessment and should generally occur at intervals of not less than 24 hours. Olanzapine can be given without regards for meals as absorption is not affected by food. Gradual tapering of the dose should be considered when discontinuing olanzapine.
A lower starting dose of 5 mg per day may be considered for geriatric patients or when other clinical factors warrant. A 5 mg starting dose also may be considered for patients with severe renal or moderate hepatic impairment.
A lower starting dose may be considered in patients who exhibit a combination of factors (female gender, geriatric age, non-smoking status) which may slow the metabolism of olanzapine. Olanzapine has not been studied in subjects under 13 years of age.
Adolescents: Schizophrenia and Acute Manic or Mixed Episodes Associated with Bipolar Disorder in Adolescents: The recommended starting dose for olanzapine is 2.5 or 5 mg administered once a day. It may be given without regard to meals as its absorption is not affected by food. The dosage range of olanzapine in adolescents is 2.5 to 20 mg per day. Daily dosage should be adjusted on the basis of clinical status. When dosage adjustments are necessary, dose increments/decrements of 2.5 or 5 mg are recommended.
Overdosage
Signs and Symptoms: Very common symptoms reported in olanzapine overdose (≥10% incidence) include tachycardia, agitation/aggressiveness, dysarthria, various extrapyramidal symptoms and reduced level of consciousness ranging from sedation to coma. Other medically significant sequelae of olanzapine overdose include delirium, convulsion, possible neuroleptic malignant syndrome, respiratory depression, aspiration, hypertension or hypotension, cardiac arrhythmias (<2% of overdose cases) and cardiopulmonary arrest. Fatal outcomes have been reported for acute overdoses as low as 450 mg of oral olanzapine but survival has also been reported following acute overdose of 2 g of oral olanzapine.
Management of Overdose: There is no specific antidote for olanzapine.
Symptomatic treatment and monitoring of vital organ function should be instituted according to clinical presentation, including treatment of hypotension and circulatory collapse and support of respiratory function. Do not use epinephrine, dopamine, or other sympathomimetic agents with beta-agonist activity since beta stimulation may worsen hypotension.
Induction of emesis is not recommended. Standard procedures for management of overdose may be indicated (i.e. gastric lavage, administration of activated charcoal). The concomitant administration of activated charcoal was shown to reduce the oral bioavailability of olanzapine by 50 to 60%.
Management of Overdose: There is no specific antidote for olanzapine.
Symptomatic treatment and monitoring of vital organ function should be instituted according to clinical presentation, including treatment of hypotension and circulatory collapse and support of respiratory function. Do not use epinephrine, dopamine, or other sympathomimetic agents with beta-agonist activity since beta stimulation may worsen hypotension.
Induction of emesis is not recommended. Standard procedures for management of overdose may be indicated (i.e. gastric lavage, administration of activated charcoal). The concomitant administration of activated charcoal was shown to reduce the oral bioavailability of olanzapine by 50 to 60%.
Administration
May be taken with or without food.
Contraindications
Olanzapine is contraindicated in patients with a known hypersensitivity to any ingredient of the product.
Warnings
Neuroleptic Malignant Syndrome (NMS): NMS, a potentially fatal symptom complex, has been reported in association with other antipsychotic drugs including olanzapine. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatinine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. Clinical manifestations of NMS or the presence of unexplained high fever without clinical manifestations of NMS require discontinuation of all antipsychotic drugs, including olanzapine.
Tardive Dyskinesia: In comparator studies with haloperidol of greater than 6 weeks, olanzapine was associated with a statistically significant lower incidence of treatment emergent dyskinesia. However, because the risk of tardive dyskinesia increases with long term exposure to antipsychotic medications, a dose reduction or drug discontinuation should be considered should signs or symptoms of tardive dyskinesia appear in a patient. These symptoms can deteriorate over time or even arise after discontinuation of treatment.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported with olanzapine exposure. DRESS consists of a combination of three or more of the following: cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, fever, lymphadenopathy and one or more systemic complications such as hepatitis, nephritis, pneumonitis, myocarditis, and pericarditis. Discontinue olanzapine if DRESS is suspected.
Safety Experience in Elderly Patients with Dementia-Related Psychosis: In elderly patients with dementia-related psychosis, the efficacy of olanzapine has not been established. In placebo-controlled clinical trials of elderly patients with dementia-related psychosis, the incidence of death in olanzapine-treated patients was significantly greater than the placebo-treated patients (3.5% vs. 1.5%, respectively). Risk factors that may predispose this patient to increased mortality when treated with olanzapine include age 80 years, sedation, concomitant use of benzodiazepines, or presence of pulmonary conditions (e.g., pneumonia, with or without aspiration).
Tardive Dyskinesia: In comparator studies with haloperidol of greater than 6 weeks, olanzapine was associated with a statistically significant lower incidence of treatment emergent dyskinesia. However, because the risk of tardive dyskinesia increases with long term exposure to antipsychotic medications, a dose reduction or drug discontinuation should be considered should signs or symptoms of tardive dyskinesia appear in a patient. These symptoms can deteriorate over time or even arise after discontinuation of treatment.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported with olanzapine exposure. DRESS consists of a combination of three or more of the following: cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, fever, lymphadenopathy and one or more systemic complications such as hepatitis, nephritis, pneumonitis, myocarditis, and pericarditis. Discontinue olanzapine if DRESS is suspected.
Safety Experience in Elderly Patients with Dementia-Related Psychosis: In elderly patients with dementia-related psychosis, the efficacy of olanzapine has not been established. In placebo-controlled clinical trials of elderly patients with dementia-related psychosis, the incidence of death in olanzapine-treated patients was significantly greater than the placebo-treated patients (3.5% vs. 1.5%, respectively). Risk factors that may predispose this patient to increased mortality when treated with olanzapine include age 80 years, sedation, concomitant use of benzodiazepines, or presence of pulmonary conditions (e.g., pneumonia, with or without aspiration).
Special Precautions
Hepatic Function Indices: Transient, asymptomatic elevations of hepatic transaminases ALT, AST have been seen occasionally, especially in early treatment. Rare postmarketing reports of hepatitis have been received. Very rare cases of cholestatic or mixed liver injury have also been reported in the postmarketing period. Caution should be exercised in patients with elevated ALT and/or AST, in patients with signs and symptoms of hepatic impairment, in patients with pre-existing conditions associated with limited hepatic functional reserve, and in patients who are being treated with potentially hepatotoxic drugs.
Hyperglycemia and Diabetes Mellitus: There is an increased prevalence of diabetes in patients with schizophrenia. As with some other antipsychotics, hyperglycemia, diabetes, exacerbation of preexisting diabetes, ketoacidosis and diabetic coma have been reported. Appropriate clinical monitoring is recommended in all patients, particularly in diabetic patients and in patients with risk factors for the development of diabetes. (See Adverse Reactions.)
Lipid Alterations: Undesirable alterations in lipids have been observed in olanzapine treated patients in placebo-controlled clinical trials. Appropriate clinical monitoring is recommended. (See Adverse Reactions.)
Cardiac Death: In a retrospective observational study, patients treated with atypical antipsychotics (including olanzapine) or typical antipsychotics had a similar dose-related increase of presumed sudden cardiac death (SCD) compared to non-users of antipsychotics (almost twice the risk than that for non-users). In postmarketing reports with olanzapine, the event of SCD has been reported very rarely.
Cerebrovascular Adverse Events (CVAE), Including Stroke, in Elderly Patients with Dementia: Cerebrovascular adverse events (e.g., stroke, transient ischemic attack), including fatalities, were reported in trials of olanzapine in elderly patients with dementia-related psychosis. In placebo-controlled studies, there was a higher incidence of CVAE in patients treated with olanzapine compared to patients treated with placebo (1.3% vs. 0.4%, respectively). All patients who experienced a cerebrovascular event had pre-existing risk factors known to be associated with an increased risk for a CVAE (e.g., history of previous CVAE or transient ischemic attack, hypertension, cigarette smoking) and presented with concurrent medical conditions and/or concomitant medications having a temporal association with CVAE. Olanzapine is not approved for the treatment of patients with dementia-related psychosis.
Seizures: Olanzapine should be used cautiously in patients who have a history of seizures or are subject to factors which may lower the seizure threshold. Seizures have been reported to occur rarely in such patients when treated with olanzapine.
Anticholinergic Activity: Experience during clinical trials revealed a low incidence of anticholinergic events. However, as clinical experience with olanzapine in patients with concomitant disease is limited, caution is advised when prescribing in patients with prostatic hypertrophy, paralytic ileus, narrow angle glaucoma, or related conditions.
Dopaminergic Antagonism: Olanzapine exhibits in vitro dopamine antagonism, and, in theory, may antagonize the effects of levodopa and dopamine agonists as with other antipsychotic drugs.
General CNS Activity: Given the primary CNS effects of olanzapine, additional caution should be used when olanzapine is taken in combination with other centrally-acting drugs, including alcohol.
Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Effects on the Ability to Drive and Use Machines: Because olanzapine may cause somnolence, patients should be cautioned about operating hazardous machinery, including motor vehicles, while taking olanzapine.
Hyperglycemia and Diabetes Mellitus: There is an increased prevalence of diabetes in patients with schizophrenia. As with some other antipsychotics, hyperglycemia, diabetes, exacerbation of preexisting diabetes, ketoacidosis and diabetic coma have been reported. Appropriate clinical monitoring is recommended in all patients, particularly in diabetic patients and in patients with risk factors for the development of diabetes. (See Adverse Reactions.)
Lipid Alterations: Undesirable alterations in lipids have been observed in olanzapine treated patients in placebo-controlled clinical trials. Appropriate clinical monitoring is recommended. (See Adverse Reactions.)
Cardiac Death: In a retrospective observational study, patients treated with atypical antipsychotics (including olanzapine) or typical antipsychotics had a similar dose-related increase of presumed sudden cardiac death (SCD) compared to non-users of antipsychotics (almost twice the risk than that for non-users). In postmarketing reports with olanzapine, the event of SCD has been reported very rarely.
Cerebrovascular Adverse Events (CVAE), Including Stroke, in Elderly Patients with Dementia: Cerebrovascular adverse events (e.g., stroke, transient ischemic attack), including fatalities, were reported in trials of olanzapine in elderly patients with dementia-related psychosis. In placebo-controlled studies, there was a higher incidence of CVAE in patients treated with olanzapine compared to patients treated with placebo (1.3% vs. 0.4%, respectively). All patients who experienced a cerebrovascular event had pre-existing risk factors known to be associated with an increased risk for a CVAE (e.g., history of previous CVAE or transient ischemic attack, hypertension, cigarette smoking) and presented with concurrent medical conditions and/or concomitant medications having a temporal association with CVAE. Olanzapine is not approved for the treatment of patients with dementia-related psychosis.
Seizures: Olanzapine should be used cautiously in patients who have a history of seizures or are subject to factors which may lower the seizure threshold. Seizures have been reported to occur rarely in such patients when treated with olanzapine.
Anticholinergic Activity: Experience during clinical trials revealed a low incidence of anticholinergic events. However, as clinical experience with olanzapine in patients with concomitant disease is limited, caution is advised when prescribing in patients with prostatic hypertrophy, paralytic ileus, narrow angle glaucoma, or related conditions.
Dopaminergic Antagonism: Olanzapine exhibits in vitro dopamine antagonism, and, in theory, may antagonize the effects of levodopa and dopamine agonists as with other antipsychotic drugs.
General CNS Activity: Given the primary CNS effects of olanzapine, additional caution should be used when olanzapine is taken in combination with other centrally-acting drugs, including alcohol.
Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Effects on the Ability to Drive and Use Machines: Because olanzapine may cause somnolence, patients should be cautioned about operating hazardous machinery, including motor vehicles, while taking olanzapine.
Use In Pregnancy & Lactation
There are no adequate and well-controlled studies with olanzapine in pregnant women. Women should be advised to notify their physicians if they become pregnant or intend to become pregnant while taking olanzapine.
Because of limited experience in humans, this drug should be used in pregnancy only when potential benefit justifies potential risk to the fetus.
In a study of lactating, healthy women, olanzapine was excreted in breast milk. Mean infant exposure (mg/Kg) at steady state was estimated to be 1.8% of the maternal olanzapine dose (mg/Kg). Patients should be advised not to breast feed an infant if they are taking olanzapine.
Because of limited experience in humans, this drug should be used in pregnancy only when potential benefit justifies potential risk to the fetus.
In a study of lactating, healthy women, olanzapine was excreted in breast milk. Mean infant exposure (mg/Kg) at steady state was estimated to be 1.8% of the maternal olanzapine dose (mg/Kg). Patients should be advised not to breast feed an infant if they are taking olanzapine.
Adverse Reactions
Adults: Weight: In clinical trials, mean weight gain was greater in patients treated with olanzapine than with placebo.
Clinically significant weight gain was observed across all baseline Body Mass Index (BMI) categories.
In long-term studies (at least 48 weeks), both the magnitude of weight gain and the proportion of olanzapine-treated patients who had clinically significant weight gain were greater than in short-term studies. The percentage of patients who gained ≥25% of their baseline body weight with long-term exposure was very common (≥10%).
Glucose: In clinical trials (up to 52 weeks) olanzapine was associated with a greater mean change in glucose relative to placebo.
The difference in mean changes between olanzapine and placebo was greater in patients with evidence of glucose dysregulation at baseline (including those patients diagnosed with diabetes mellitus or who met criteria suggestive of hyperglycemia), and these patients had a greater increase in HbA1c compared to placebo.
The proportion of patients who had a change in glucose level from normal or borderline at baseline to high increased over time. In an analysis of patients who completed 9-12 months of olanzapine therapy, the rate of increase in mean blood glucose slowed after approximately 6 months.
Lipids: In clinical trials of up to 12 weeks in duration, olanzapine-treated patients had a greater mean increase in fasting total cholesterol, LDL cholesterol, and triglycerides, compared to placebo-treated patients.
Mean increases in fasting lipid values (total cholesterol, LDL cholesterol, and triglycerides) were greater in patients without evidence of lipid dysregulation at baseline. For fasting HDL cholesterol, no statistically significant differences were observed between olanzapine-treated patients and placebo-treated patients.
The proportion of patients who had changes in total cholesterol, LDL cholesterol or triglycerides from normal or borderline to high, or changes in HDL cholesterol from normal or borderline to low, was greater in long-term studies (at least 48 weeks) as compared with short-term studies. In an analysis of patients who completed 12 months of therapy, the mean nonfasting total cholesterol did not increase further after approximately 4-6 months.
Prolactin: In controlled clinical trials (up to 12 weeks), elevations in prolactin were observed in 30% of olanzapine-treated patients as compared to 10.5% of placebo-treated patients. In the majority of these patients, the elevations were mild.
In patients with schizophrenia, menstrual-related adverse events potentially associated with prolactin elevations were common (<10% to =1%), whereas sexual function-related and breast-related adverse events were infrequent (<1% to =0.1%). In patients treated for other mental illnesses, sexual function-related adverse events potentially associated with prolactin elevations were common (<10% to =1%), whereas breast-related and menstrual-related adverse events were infrequent (<1% to =0.1%).
(1) TEAEs analysis up to 52 weeks of treatment.
(2) Bipolar Depression, Psychotic Depression, Borderline Personality Disorder and Bipolar Mania.
Hepatic Aminotransferases: Transient, asymptomatic elevations of hepatic transaminases, ALT/SGPT and AST/SGOT, have been seen occasionally.
Eosinophilia: Asymptomatic eosinophilia was occasionally seen.
Undesirable Effects for Special Population: Very common (≥ 10%) undesirable effects associated with the use of Olanzapine in clinical trials with elderly patients with dementia-related psychosis were abnormal gait and falls.
Common (<10% and ≥1%) undesirable effects associated with the use of olanzapine in elderly patients with dementia-related psychosis were urinary incontinence and pneumonia.
In clinical trials in patients with drug-induced (dopamine agonist) psychosis associated with Parkinson's disease, worsening of Parkinsonian symptomatology was reported very commonly and more frequently than with placebo. Also, hallucinations were reported very commonly and more frequently than with placebo. In these trials, patients were required to be stable on the lowest effective dose of anti-Parkinsonian medications (dopamine agonist) prior to the beginning of the study and to remain on the same anti-Parkinsonian medications and dosages throughout the study. Olanzapine was started at 2.5 mg/day and titrated up to a maximum of 15 mg/day based on the investigator judgement.
The following table summarizes the core adverse drug reaction terms and their frequencies identified during clinical trials and/or during post-marketing experience for olanzapine. (See Tables 2a and 2b.)
Clinically significant weight gain was observed across all baseline Body Mass Index (BMI) categories.
In long-term studies (at least 48 weeks), both the magnitude of weight gain and the proportion of olanzapine-treated patients who had clinically significant weight gain were greater than in short-term studies. The percentage of patients who gained ≥25% of their baseline body weight with long-term exposure was very common (≥10%).
Glucose: In clinical trials (up to 52 weeks) olanzapine was associated with a greater mean change in glucose relative to placebo.
The difference in mean changes between olanzapine and placebo was greater in patients with evidence of glucose dysregulation at baseline (including those patients diagnosed with diabetes mellitus or who met criteria suggestive of hyperglycemia), and these patients had a greater increase in HbA1c compared to placebo.
The proportion of patients who had a change in glucose level from normal or borderline at baseline to high increased over time. In an analysis of patients who completed 9-12 months of olanzapine therapy, the rate of increase in mean blood glucose slowed after approximately 6 months.
Lipids: In clinical trials of up to 12 weeks in duration, olanzapine-treated patients had a greater mean increase in fasting total cholesterol, LDL cholesterol, and triglycerides, compared to placebo-treated patients.
Mean increases in fasting lipid values (total cholesterol, LDL cholesterol, and triglycerides) were greater in patients without evidence of lipid dysregulation at baseline. For fasting HDL cholesterol, no statistically significant differences were observed between olanzapine-treated patients and placebo-treated patients.
The proportion of patients who had changes in total cholesterol, LDL cholesterol or triglycerides from normal or borderline to high, or changes in HDL cholesterol from normal or borderline to low, was greater in long-term studies (at least 48 weeks) as compared with short-term studies. In an analysis of patients who completed 12 months of therapy, the mean nonfasting total cholesterol did not increase further after approximately 4-6 months.
Prolactin: In controlled clinical trials (up to 12 weeks), elevations in prolactin were observed in 30% of olanzapine-treated patients as compared to 10.5% of placebo-treated patients. In the majority of these patients, the elevations were mild.
In patients with schizophrenia, menstrual-related adverse events potentially associated with prolactin elevations were common (<10% to =1%), whereas sexual function-related and breast-related adverse events were infrequent (<1% to =0.1%). In patients treated for other mental illnesses, sexual function-related adverse events potentially associated with prolactin elevations were common (<10% to =1%), whereas breast-related and menstrual-related adverse events were infrequent (<1% to =0.1%).
(1) TEAEs analysis up to 52 weeks of treatment.
(2) Bipolar Depression, Psychotic Depression, Borderline Personality Disorder and Bipolar Mania.
Hepatic Aminotransferases: Transient, asymptomatic elevations of hepatic transaminases, ALT/SGPT and AST/SGOT, have been seen occasionally.
Eosinophilia: Asymptomatic eosinophilia was occasionally seen.
Undesirable Effects for Special Population: Very common (≥ 10%) undesirable effects associated with the use of Olanzapine in clinical trials with elderly patients with dementia-related psychosis were abnormal gait and falls.
Common (<10% and ≥1%) undesirable effects associated with the use of olanzapine in elderly patients with dementia-related psychosis were urinary incontinence and pneumonia.
In clinical trials in patients with drug-induced (dopamine agonist) psychosis associated with Parkinson's disease, worsening of Parkinsonian symptomatology was reported very commonly and more frequently than with placebo. Also, hallucinations were reported very commonly and more frequently than with placebo. In these trials, patients were required to be stable on the lowest effective dose of anti-Parkinsonian medications (dopamine agonist) prior to the beginning of the study and to remain on the same anti-Parkinsonian medications and dosages throughout the study. Olanzapine was started at 2.5 mg/day and titrated up to a maximum of 15 mg/day based on the investigator judgement.
The following table summarizes the core adverse drug reaction terms and their frequencies identified during clinical trials and/or during post-marketing experience for olanzapine. (See Tables 2a and 2b.)
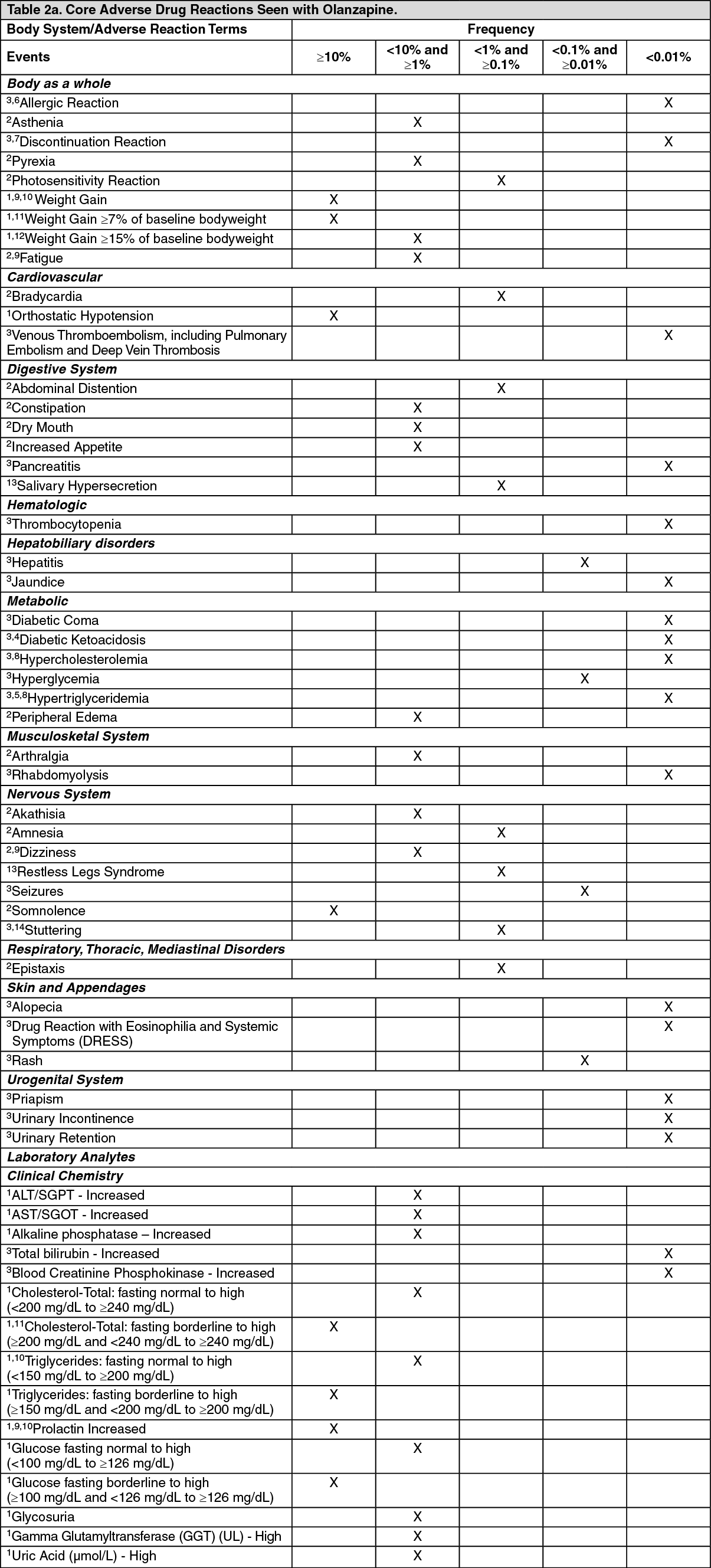

Adolescents (Ages 13-17 Years): The types of undesirable effects observed in adolescent patients treated with olanzapine were similar to those seen in adult patients. Although no clinical trials designed to compare adolescents to adults were conducted, the data from the adolescent trials were compared to those of the adult trials.
Mean increase in weight in adolescents (4.6 kg over 3 weeks median duration of exposure) was greater than in adults (2.6 kg over 7 weeks median duration of exposure).
In long-term studies (at least 24 weeks), both the magnitude of weight gain and the proportion of adolescent olanzapine-treated patients who had clinically significant weight gain were greater than in short-term studies and were greater than in adult patients with comparable exposures. With long-term exposure, approximately half of adolescent patients gained ≥15% and almost a third gained ≥25% of their baseline body weight. Among adolescent patients, mean weight gain was greatest in patients who were overweight or obese at baseline.
Mean increases in fasting glucose were similar in adolescents and adults treated with olanzapine, however, the difference between olanzapine and placebo groups was greater in adolescents compared to adults.
In long-term studies (at least 24 weeks), changes in glucose from normal at baseline to high were uncommon (<1% and ≥0.1%).
Mean increases in fasting total cholesterol, LDL cholesterol, and triglycerides were generally greater in adolescents than in adults treated with olanzapine. However, in short term trials the differences between olanzapine and placebo were similar for adolescents and adults.
Adolescents treated with olanzapine experienced a significantly higher incidence of elevated prolactin levels and significantly higher mean increases in prolactin levels compared with adults.
Hepatic aminotransferase elevations are more common in adolescents as compared to adults. Sedation-related events are more common in adolescents as compared to adults.
The following table summarizes core adverse drug reaction terms and their frequencies identified only during clinical trials in adolescent patients (13 to 17 years):See Table 3.
Mean increase in weight in adolescents (4.6 kg over 3 weeks median duration of exposure) was greater than in adults (2.6 kg over 7 weeks median duration of exposure).
In long-term studies (at least 24 weeks), both the magnitude of weight gain and the proportion of adolescent olanzapine-treated patients who had clinically significant weight gain were greater than in short-term studies and were greater than in adult patients with comparable exposures. With long-term exposure, approximately half of adolescent patients gained ≥15% and almost a third gained ≥25% of their baseline body weight. Among adolescent patients, mean weight gain was greatest in patients who were overweight or obese at baseline.
Mean increases in fasting glucose were similar in adolescents and adults treated with olanzapine, however, the difference between olanzapine and placebo groups was greater in adolescents compared to adults.
In long-term studies (at least 24 weeks), changes in glucose from normal at baseline to high were uncommon (<1% and ≥0.1%).
Mean increases in fasting total cholesterol, LDL cholesterol, and triglycerides were generally greater in adolescents than in adults treated with olanzapine. However, in short term trials the differences between olanzapine and placebo were similar for adolescents and adults.
Adolescents treated with olanzapine experienced a significantly higher incidence of elevated prolactin levels and significantly higher mean increases in prolactin levels compared with adults.
Hepatic aminotransferase elevations are more common in adolescents as compared to adults. Sedation-related events are more common in adolescents as compared to adults.
The following table summarizes core adverse drug reaction terms and their frequencies identified only during clinical trials in adolescent patients (13 to 17 years):See Table 3.
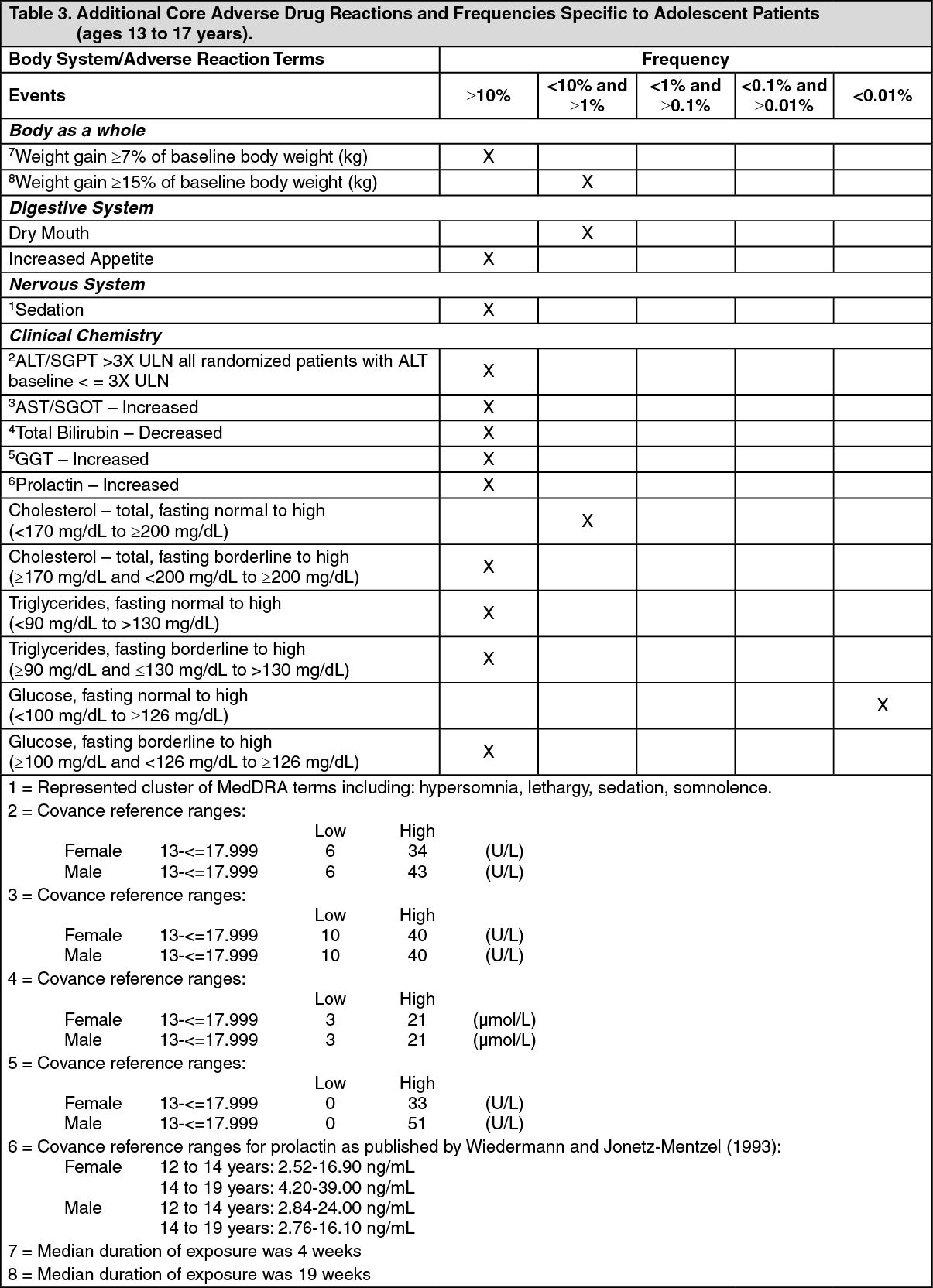
The following table summarizes additional core adverse drug reaction terms and their frequencies identified only during clinical trials in patients with dementia of the Alzheimer's type: See Table 4.
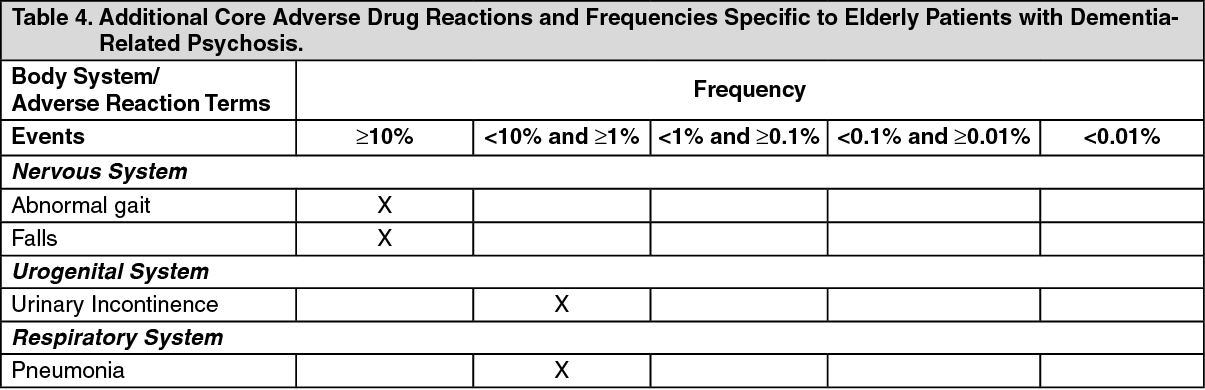
The following table summarizes additional core adverse drug reaction terms and their frequencies identified only during clinical trials in patients with drug-induced (dopamine agonist) psychosis associated with Parkinson's Disease: See Table 5.
The following table summarizes additional core adverse drug reaction terms and their frequencies identified only during bipolar mania clinical trials in patients receiving olanzapine in combination with lithium or valproate: See Table 6.

Drug Interactions
The metabolism of olanzapine may be affected by inhibitors or inducers of the P450 cytochrome isoforms, specifically CYP1A2 activity. Olanzapine clearance was increased by smoking or carbamazepine coadministration. Smoking and carbamazepine therapy are known to induce CYP1A2 activity. Known potent inhibitors of CYP1A2 activity may decrease olanzapine clearance. Olanzapine is not a potent inhibitor of CYP1A2 activity. The pharmacokinetics of theophylline, a drug principally metabolized by CYP1A2, are not altered by olanzapine.
The following drugs were given with single doses of olanzapine in clinical trials and showed no inhibition of metabolism: imipramine or its metabolite desipramine (CYP2D6, CYP3A, CYP1A2), warfarin (CYP2C19), theophylline (CYP1A2), or diazepam (CYP3A4, CYP2C19). Olanzapine also showed no interaction when administered with lithium or biperiden.
Steady state concentrations of olanzapine had no effect on the pharmacokinetics of ethanol. However, additive pharmacological effects such as increased sedation may occur when ethanol is ingested together with olanzapine.
Fluoxetine (60 mg single dose or 60 mg daily for 8 days) causes a mean 16% increase in the maximum concentration of olanzapine and a mean 16% decrease in olanzapine clearance. The magnitude of the impact of this factor is small in comparison to the overall variability between individuals, and therefore dose modification is not routinely recommended.
Fluvoxamine, a CYP1A2 inhibitor, decreases the clearance of olanzapine. This results in a mean increase in olanzapine Cmax following fluvoxamine of 54% in female non-smokers and 77% in male smokers. The mean increase in olanzapine AUC is 52% and 108%, respectively. Lower doses of olanzapine should be considered in patients receiving concomitant treatment with fluvoxamine.
Studies in vitro using human liver microsomes showed that olanzapine has little potential to inhibit the glucuronidation of valproate, which is the major metabolic pathway. Furthermore, valproate was found to have little effect on the metabolism of olanzapine in vitro. Daily concomitant in vivo administration of 10 mg olanzapine for 2 weeks did not affect steady state plasma concentration of valproate. Therefore, concomitant olanzapine administration does not require dosage adjustment of valproate.
Olanzapine absorption is not affected by food.
The effect of drugs likely to change absorption of oral olanzapine were also studied.
A single dose of an aluminum-and magnesium-containing antacid or cimetidine did not affect oral bioavailability of olanzapine. Concomitant administration of activated charcoal reduced oral bioavailability of olanzapine by 50 to 60%.
The following drugs were given with single doses of olanzapine in clinical trials and showed no inhibition of metabolism: imipramine or its metabolite desipramine (CYP2D6, CYP3A, CYP1A2), warfarin (CYP2C19), theophylline (CYP1A2), or diazepam (CYP3A4, CYP2C19). Olanzapine also showed no interaction when administered with lithium or biperiden.
Steady state concentrations of olanzapine had no effect on the pharmacokinetics of ethanol. However, additive pharmacological effects such as increased sedation may occur when ethanol is ingested together with olanzapine.
Fluoxetine (60 mg single dose or 60 mg daily for 8 days) causes a mean 16% increase in the maximum concentration of olanzapine and a mean 16% decrease in olanzapine clearance. The magnitude of the impact of this factor is small in comparison to the overall variability between individuals, and therefore dose modification is not routinely recommended.
Fluvoxamine, a CYP1A2 inhibitor, decreases the clearance of olanzapine. This results in a mean increase in olanzapine Cmax following fluvoxamine of 54% in female non-smokers and 77% in male smokers. The mean increase in olanzapine AUC is 52% and 108%, respectively. Lower doses of olanzapine should be considered in patients receiving concomitant treatment with fluvoxamine.
Studies in vitro using human liver microsomes showed that olanzapine has little potential to inhibit the glucuronidation of valproate, which is the major metabolic pathway. Furthermore, valproate was found to have little effect on the metabolism of olanzapine in vitro. Daily concomitant in vivo administration of 10 mg olanzapine for 2 weeks did not affect steady state plasma concentration of valproate. Therefore, concomitant olanzapine administration does not require dosage adjustment of valproate.
Olanzapine absorption is not affected by food.
The effect of drugs likely to change absorption of oral olanzapine were also studied.
A single dose of an aluminum-and magnesium-containing antacid or cimetidine did not affect oral bioavailability of olanzapine. Concomitant administration of activated charcoal reduced oral bioavailability of olanzapine by 50 to 60%.
Caution For Usage
Major Incompatibilities: None.
Instructions for Use/Handling: Use as directed by physician.
Instructions for Use/Handling: Use as directed by physician.
Storage
Protect from light and moisture. Store at temperatures not exceeding 30°C.
Shelf Life: Three years when stored under appropriate conditions.
Shelf Life: Three years when stored under appropriate conditions.
Action
Therapeutic Class: Olanzapine is an antipsychotic agent.
Pharmacology: Pharmacodynamics: Olanzapine is an antipsychotic agent that demonstrates a broad pharmacologic profile across a number of receptor systems. In preclinical studies, olanzapine exhibited affinities for serotonin 5-HT2A/C, 5 HT3, 5 HT6; dopamine D1, D2, D3, D4, D5; muscarinic M1.5; adrenergic α1; and histamine H1, receptors. Animal behavioral studies with olanzapine indicated 5HT, dopamine, and cholinergic antagonism, consistent with the receptor-binding profile. Olanzapine demonstrated greater in vitro receptor affinity for serotonin 5HT2, as well as greater in vivo serotonin 5HT2 activity compared to dopamine D2 receptor affinity and activity. Electrophysiological studies demonstrated that olanzapine selectively reduced the firing of mesolimbic (A10) dopaminergic neurons, while having little effect on the striatal (A9) pathways involved in motor function. Olanzapine reduced a conditioned avoidance response, a test indicative of antipsychotic activity, at doses below those producing catalepsy, an effect indicative of motor side-effects. Unlike some other antipsychotic agents, olanzapine increases responding in an "anxiolytic" test.
In two of two placebo and two of three comparator-controlled trials with over 2,900 schizophrenic patients presenting with both positive and negative symptoms, olanzapine was associated with statistically significantly greater improvements in negative as well as positive symptoms.
Pharmacokinetics: Olanzapine is well absorbed after oral administration, reaching peak plasma concentrations within 5 to 8 hours. The absorption is not affected by food. Plasma concentrations of olanzapine were linear and dose proportional in trials studying doses from 1 to 20 mg.
Olanzapine is metabolized in the liver by conjugative and oxidative pathways. The major circulating metabolite is the 10-N-glucuronide, which in theory does not pass the blood-brain barrier. Cytochrome P450 isoforms CYP1A2 and CYP2D6 contribute to the formation of the N-desmethyl and 2-hydroxymethyl metabolites. Both metabolites exhibited significantly less in vivo pharmacological activity than olanzapine in animal studies. The predominant pharmacologic activity is from the parent olanzapine.
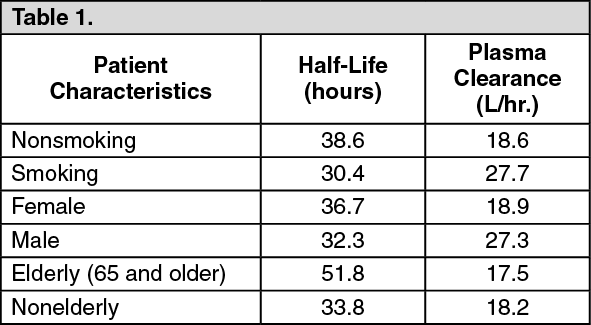
Although smoking status, gender, and, to a lesser extent, age may affect olanzapine clearance and half-life, the magnitude of the impact of these single factors is small in comparison to the overall variability between individuals.
The plasma protein binding of olanzapine was about 93% over the concentration range of about 7 to about 1000 ng/mL. Olanzapine is bound predominantly to albumin, and α1-acid-glycoprotein.
After oral administration to healthy subjects, the mean terminal elimination half-life was 33 hours (21 to 54 hours for 5th to 95th percentile) and the mean olanzapine plasma clearance was 26 L/hr (12 to 47 L/hr for the 5th to 95th percentile). Olanzapine pharmacokinetics varied on the basis of smoking status, gender, and age.
The following table summarizes these effects: See Table 1.
Pharmacology: Pharmacodynamics: Olanzapine is an antipsychotic agent that demonstrates a broad pharmacologic profile across a number of receptor systems. In preclinical studies, olanzapine exhibited affinities for serotonin 5-HT2A/C, 5 HT3, 5 HT6; dopamine D1, D2, D3, D4, D5; muscarinic M1.5; adrenergic α1; and histamine H1, receptors. Animal behavioral studies with olanzapine indicated 5HT, dopamine, and cholinergic antagonism, consistent with the receptor-binding profile. Olanzapine demonstrated greater in vitro receptor affinity for serotonin 5HT2, as well as greater in vivo serotonin 5HT2 activity compared to dopamine D2 receptor affinity and activity. Electrophysiological studies demonstrated that olanzapine selectively reduced the firing of mesolimbic (A10) dopaminergic neurons, while having little effect on the striatal (A9) pathways involved in motor function. Olanzapine reduced a conditioned avoidance response, a test indicative of antipsychotic activity, at doses below those producing catalepsy, an effect indicative of motor side-effects. Unlike some other antipsychotic agents, olanzapine increases responding in an "anxiolytic" test.
In two of two placebo and two of three comparator-controlled trials with over 2,900 schizophrenic patients presenting with both positive and negative symptoms, olanzapine was associated with statistically significantly greater improvements in negative as well as positive symptoms.
Pharmacokinetics: Olanzapine is well absorbed after oral administration, reaching peak plasma concentrations within 5 to 8 hours. The absorption is not affected by food. Plasma concentrations of olanzapine were linear and dose proportional in trials studying doses from 1 to 20 mg.
Olanzapine is metabolized in the liver by conjugative and oxidative pathways. The major circulating metabolite is the 10-N-glucuronide, which in theory does not pass the blood-brain barrier. Cytochrome P450 isoforms CYP1A2 and CYP2D6 contribute to the formation of the N-desmethyl and 2-hydroxymethyl metabolites. Both metabolites exhibited significantly less in vivo pharmacological activity than olanzapine in animal studies. The predominant pharmacologic activity is from the parent olanzapine.
Although smoking status, gender, and, to a lesser extent, age may affect olanzapine clearance and half-life, the magnitude of the impact of these single factors is small in comparison to the overall variability between individuals.
The plasma protein binding of olanzapine was about 93% over the concentration range of about 7 to about 1000 ng/mL. Olanzapine is bound predominantly to albumin, and α1-acid-glycoprotein.
After oral administration to healthy subjects, the mean terminal elimination half-life was 33 hours (21 to 54 hours for 5th to 95th percentile) and the mean olanzapine plasma clearance was 26 L/hr (12 to 47 L/hr for the 5th to 95th percentile). Olanzapine pharmacokinetics varied on the basis of smoking status, gender, and age.
The following table summarizes these effects: See Table 1.
Adolescents (ages 13 to 17 years): The pharmacokinetics of olanzapine are similar between adolescents and adults. In clinical studies, the average olanzapine exposure was approximately 27% higher in adolescents. Demographic differences between the adolescents and adults include a lower average body weight and fewer adolescents were smokers. Such factors likely contribute to the higher average exposure observed in adolescents.
There was no significant difference in mean elimination half-life or olanzapine plasma clearance between subjects with severely impaired renal function compared to individuals with normal renal function. Approximately 57% of radio-labeled olanzapine is excreted in urine, principally as metabolites.
Subjects with mild hepatic dysfunction who smoked had reduced clearance comparable to nonsmoking subjects with no hepatic dysfunction.
In a study of Caucasians, Japanese, and Chinese subjects, there were no differences in olanzapine pharmacokinetics among the three populations. Cytochrome P450 isoform CYP2D6 status does not affect the metabolism of olanzapine.
Toxicology: Carcinogenesis, Mutagenesis, Impairment of Fertility, Animal Toxicity: Based on results of studies in rats and mice, it was concluded that olanzapine is not carcinogenic. Significant findings in oncogenicity studies were limited to an increased incidence of mammary adenocarcinomas in female rats and mice. This is a common finding in rodents treated with agents that increase prolactin secretion and has no direct significance for humans.
Olanzapine was not mutagenic in a full range of standard tests, which included bacterial mutation tests and in vitro and in vivo mammalian tests.
In animal studies, olanzapine had no teratogenic effects. Sedation affected mating performance of male rats. Estrous cycles were affected at doses of 1.1 mg/kg (3 times the maximum human dose) and reproduction parameters were influenced in rats given 3mg/kg (9 times the maximum human dose). In the offspring of rats given olanzapine, delays in fetal development and transient decreases in offspring activity levels were seen.
In animal studies with olanzapine, the principal hematologic findings were reversible peripheral cytopenias in individual dogs given high doses of olanzapine (24 to 30 times the maximum daily human dose), dose-related decreases in lymphocytes and neutrophils in mice, and lymphopenia secondary to compromised nutritional status in rats. A few dogs treated with 24 to 30 times the maximum daily human dose developed reversible neutropenia or reversible hemolytic anemia between 1 and 10 months of treatment. Effects on hematology parameters in each species involved circulating blood cells, and no evidence of bone marrow cytotoxicity was found in any of the species examined.
There was no significant difference in mean elimination half-life or olanzapine plasma clearance between subjects with severely impaired renal function compared to individuals with normal renal function. Approximately 57% of radio-labeled olanzapine is excreted in urine, principally as metabolites.
Subjects with mild hepatic dysfunction who smoked had reduced clearance comparable to nonsmoking subjects with no hepatic dysfunction.
In a study of Caucasians, Japanese, and Chinese subjects, there were no differences in olanzapine pharmacokinetics among the three populations. Cytochrome P450 isoform CYP2D6 status does not affect the metabolism of olanzapine.
Toxicology: Carcinogenesis, Mutagenesis, Impairment of Fertility, Animal Toxicity: Based on results of studies in rats and mice, it was concluded that olanzapine is not carcinogenic. Significant findings in oncogenicity studies were limited to an increased incidence of mammary adenocarcinomas in female rats and mice. This is a common finding in rodents treated with agents that increase prolactin secretion and has no direct significance for humans.
Olanzapine was not mutagenic in a full range of standard tests, which included bacterial mutation tests and in vitro and in vivo mammalian tests.
In animal studies, olanzapine had no teratogenic effects. Sedation affected mating performance of male rats. Estrous cycles were affected at doses of 1.1 mg/kg (3 times the maximum human dose) and reproduction parameters were influenced in rats given 3mg/kg (9 times the maximum human dose). In the offspring of rats given olanzapine, delays in fetal development and transient decreases in offspring activity levels were seen.
In animal studies with olanzapine, the principal hematologic findings were reversible peripheral cytopenias in individual dogs given high doses of olanzapine (24 to 30 times the maximum daily human dose), dose-related decreases in lymphocytes and neutrophils in mice, and lymphopenia secondary to compromised nutritional status in rats. A few dogs treated with 24 to 30 times the maximum daily human dose developed reversible neutropenia or reversible hemolytic anemia between 1 and 10 months of treatment. Effects on hematology parameters in each species involved circulating blood cells, and no evidence of bone marrow cytotoxicity was found in any of the species examined.
MedsGo Class
Antipsychotics
Features
Dosage
10mg
Ingredients
- Olanzapine
Packaging
Film-Coated Tablet 1's
Generic Name
Olanzapine
Registration Number
DR-XY22856
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions


