All variations
Indications / Uses
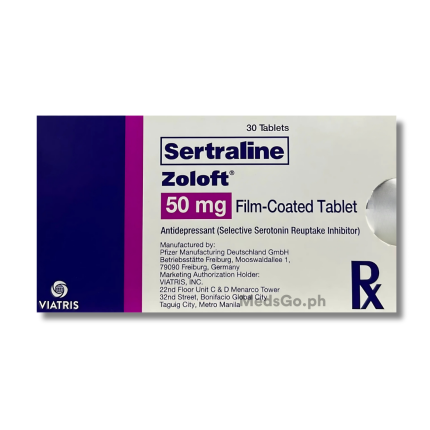
ZOLOFT Sertraline 50mg is used for the management and treatment of various mood and anxiety disorders in adults. This includes relief for symptoms associated with major depressive disorder, obsessive-compulsive disorder (OCD), and panic disorder, with or without accompanying agoraphobia. It is also effective in managing post-traumatic stress disorder (PTSD), social phobia, and premenstrual dysphoric disorder (PMDD). The medication works by helping to restore the balance of certain natural substances in the brain.
Formulation / Ingredients
ZOLOFT Sertraline 50mg contains: Sertraline (as hydrochloride) 50mg
Do I need prescription to buy ZOLOFT Sertraline 50mg?
Yes, you need a prescription. But if you don't have one, you can get a free teleconsultation from MedsGo Physician. Just make an order and our Physician will call you back.
Side Effects
ZOLOFT Sertraline 50mg common side effects include: gastrointestinal disturbances like nausea and diarrhea, dry mouth, excessive sweating, and sleep pattern changes such as insomnia or drowsiness. Fatigue and dizziness are also frequently reported during treatment initiation. Other notable, less common effects may include decreased sexual drive, or issues with ejaculation.
Directions and Dosage
- Dosage for Adults (18+ years) for Depression and OCD: Initial dose is 50 mg once daily. Dosage may be gradually increased in increments up to a maximum of 200 mg daily.
- Dosage for Adults (18+ years) for Panic Disorder, PTSD, and Social Phobia: Initial dose is 25 mg once daily. After 1 week, the dose should be increased to 50 mg once daily. Maximum dosage is 200 mg daily.
- Dosage for Adolescents (13–17 years) for OCD: Initial dose is 50 mg once daily. Maximum dosage is 200 mg daily.
- Dosage for Kids 6–12 years old for OCD: Initial dose is 25 mg once daily. After 1 week, the dose should be increased to 50 mg once daily. Maximum dosage is 200 mg daily.
The medication is not recommended for kids under 6 years of age. An observable effect may be seen within 7 days, but full therapeutic activity typically requires 2 to 4 weeks, and potentially longer for certain conditions.
Contraindications
This medication is contraindicated if there is a known sensitivity to its active substance or any of its components. It must not be taken concurrently with or within 14 days of discontinuing another class of medicines used for mood disorders, known as Monoamine Oxidase Inhibitors. Concomitant use with certain antipsychotic medicines is also prohibited.
Use in Pregnancy and Lactation
The use of this medication during pregnancy is generally not recommended unless the potential benefits significantly outweigh any risks. Use in the later stages of pregnancy, particularly the third trimester, may be associated with complications in the newborn, including breathing difficulties and withdrawal reactions. Furthermore, some data suggests an increased risk of severe bleeding after delivery and a risk of pulmonary complications in the neonate. Small amounts of the active substance are found in breast milk. Use while nursing is not recommended unless the benefit to the woman is greater than the potential risks to the infant.
Special Precautions
- Close monitoring is necessary for any worsening of mood or unusual behavioral changes, particularly during the start of therapy or with dose changes.
- Use with caution if there is a history of seizures or epilepsy, as well as in individuals with a tendency towards increased bleeding or bruising.
- Dosing adjustments may be required for those with existing liver function impairment.
- Caution is advised for people with pre-existing heart conditions that may cause irregular heartbeats, or for those with diabetes or glaucoma.
- Periodic checks for low sodium levels in the blood are advisable, especially in older individuals.
Is it safe to take it with other medications?
Combining this substance with other medications that increase serotonin levels can heighten the risk of Serotonin Syndrome. Caution is also necessary if taking nonsteroidal anti-inflammatory drugs (NSAIDs) or blood thinning agents, as this combination may elevate the risk of abnormal bleeding or bruising. Avoid concurrent use with certain antipsychotic medicines.
How should I store it?
Store the product in its original packaging at temperatures not exceeding 30°C. Ensure it is protected from moisture and light to maintain its effectiveness.
Used For
- Depression, OCD, Anxiety
Age
- 6 years & up
Features
- Sertraline
Reviews
No reviews found
Product Questions