All variations
Indications/Uses
Neuropathic pain: Pregabalin is indicated for the treatment of peripheral and central neuropathic pain in adults.
Epilepsy: Pregabalin is indicate as adjunctive therapy in adults with partial seizures with or without secondary generalization.
Adjunctive therapy for adult patients with partial onset seizures.
Generalised Anxiety Disorder: Pregabalin is indicated for treatment of Generalised Anxiety Disorder (GAD) in adults.
Epilepsy: Pregabalin is indicate as adjunctive therapy in adults with partial seizures with or without secondary generalization.
Adjunctive therapy for adult patients with partial onset seizures.
Generalised Anxiety Disorder: Pregabalin is indicated for treatment of Generalised Anxiety Disorder (GAD) in adults.
Dosage/Direction for Use
The dose range is 150 to 600 mg per day given in either two or three divided doses.
Neuropathic pain: Pregabalin treatment can be started at a dose of 150 mg per day given as two or three divided doses. Based on individual patient response and tolerability, the dose may be increased to 300 mg per day after an interval of 3 to 7 days, and if needed, to a maximum dose of 600 mg per day after an additional 7-day interval.
Epilepsy: Pregabalin treatment can be started with a dose of 150 mg per day given as two or three divided doses. Based on individual patient response and tolerability, the dose may be increased to 300 mg per day after 1 week. The maximum dose of 600 mg per day may be achieved after an additional week.
Generalised Anxiety Disorder: The dose range is 150 to 600 mg per day given as two or three divided doses. The need for treatment should be reassessed regularly.
Pregabalin treatment can be started with a dose of 150 mg per day. Based on individual patient response and tolerability, the dose may be increased to 300 mg per day after 1 week. Following an additional week the dose may be increased to 450 mg per day. The maximum dose of 600 mg per day may be achieved after an additional week.
Discontinuation of pregabalin: In accordance with current clinical practice, if pregabalin has to be discontinued it is recommended this should be done gradually over a minimum of 1 week independent of the indication.
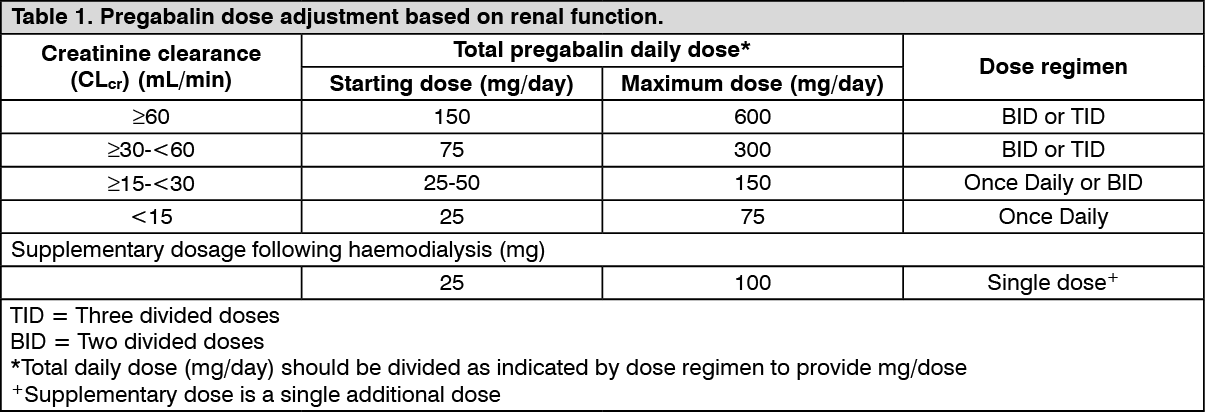
Special populations: Patients with renal impairment: Pregabalin is eliminated from the systemic circulation primarily by renal excretion as unchanged drug. As pregabalin clearance is directly proportional to creatinine clearance, dose reduction in patients with compromised renal function must be individualised according to creatinine clearance (CLcr), as indicated in Table 1 determined using the following formula: See formula.
Neuropathic pain: Pregabalin treatment can be started at a dose of 150 mg per day given as two or three divided doses. Based on individual patient response and tolerability, the dose may be increased to 300 mg per day after an interval of 3 to 7 days, and if needed, to a maximum dose of 600 mg per day after an additional 7-day interval.
Epilepsy: Pregabalin treatment can be started with a dose of 150 mg per day given as two or three divided doses. Based on individual patient response and tolerability, the dose may be increased to 300 mg per day after 1 week. The maximum dose of 600 mg per day may be achieved after an additional week.
Generalised Anxiety Disorder: The dose range is 150 to 600 mg per day given as two or three divided doses. The need for treatment should be reassessed regularly.
Pregabalin treatment can be started with a dose of 150 mg per day. Based on individual patient response and tolerability, the dose may be increased to 300 mg per day after 1 week. Following an additional week the dose may be increased to 450 mg per day. The maximum dose of 600 mg per day may be achieved after an additional week.
Discontinuation of pregabalin: In accordance with current clinical practice, if pregabalin has to be discontinued it is recommended this should be done gradually over a minimum of 1 week independent of the indication.
Special populations: Patients with renal impairment: Pregabalin is eliminated from the systemic circulation primarily by renal excretion as unchanged drug. As pregabalin clearance is directly proportional to creatinine clearance, dose reduction in patients with compromised renal function must be individualised according to creatinine clearance (CLcr), as indicated in Table 1 determined using the following formula: See formula.
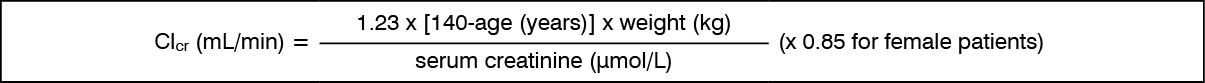
Pregabalin is removed effectively from plasma by haemodialysis (50% of drug in 4 hours). For patients receiving haemodialysis, the pregabalin daily dose should be adjusted based on renal function. In addition to the daily dose, a supplementary dose should be given immediately following every 4-hour haemodialysis treatment. (See Table 1.)
Paediatric population: The safety and efficacy of Pregabalin Capsules in children below the age of 12 years and in adolescents (12-17 years of age) have not been established. No data are available.
Use in the elderly (over 65 years of age): Elderly patients may require a dose reduction of pregabalin due to a decreased renal function (see patients with renal impairment).
Method of administration: Pregabalin Capsules may be taken with or without food.
Pregabalin Capsules are for oral use only.
Use in the elderly (over 65 years of age): Elderly patients may require a dose reduction of pregabalin due to a decreased renal function (see patients with renal impairment).
Method of administration: Pregabalin Capsules may be taken with or without food.
Pregabalin Capsules are for oral use only.
Overdosage
In overdoses up to 15g, no unexpected adverse reactions were reported. The most adverse reactions observed when pregabalin was taken in overdose included somnolence, confusional state, agitation, and restlessness.
Treatment of pregabalin overdose should include general supportive measures and may include haemodialysis if necessary.
Treatment of pregabalin overdose should include general supportive measures and may include haemodialysis if necessary.
Administration
May be taken with or without food.
Contraindications
VEXER is contraindicated in patients with known hypersensitivity to pregabalin or any of its other components.
Warnings
Angioedema: There have been postmarketing reports of angioedema in patients during initial and chronic treatment with Pregabalin. Specific symptoms included swelling of the face, mouth (tongue, lips, and gums), and neck (throat and larynx). There were reports of life-threatening angioedema with respiratory compromise requiring emergency treatment. Pregabalin should be discontinued immediately in patients with these symptoms.
Caution should be exercised when prescribing Pregabalin to patients who have had a previous episode of angioedema. In addition, patients who are taking other drugs associated with angioedema (e.g., angiotensin converting enzyme inhibitors [ACE-inhibitors]) may be at increased risk of developing angioedema.
Hypersensitivity: There have been postmarketing reports of hypersensitivity in patients shortly after initiation of treatment with pregabalin. Adverse reactions included skin redness, blisters, hives, rash, dyspnea, and wheezing. Pregabalin should be discontinued immediately in patients with these symptoms.
Withdrawal of Antiepileptic Drugs (AEDs): As with all AEDs, pregabalin should be withdrawn gradually to minimize the potential of increased seizure frequency in patients with seizure disorders. If pregabalin is discontinued this should be done gradually over a minimum of 1 week.
Pregabalin treatment may cause peripheral edema. In short-term trials of patients without clinically significant heart or peripheral vascular disease, there was no apparent association between peripheral edema and cardiovascular complications such as hypertension or congestive heart failure. Peripheral edema was not associated with laboratory changes suggestive of deterioration in renal or hepatic function.
As the thiazolidinedione class of antidiabetic drugs can cause weight gain and/or fluid retention, possibly exacerbating or leading to heart failure, care should be taken when co-administering VEXER and these agents.
Dizziness and Somnolence: VEXER may cause dizziness and somnolence. Patients should be informed that pregabalin-related dizziness and somnolence may impair their ability to perform tasks such as driving or operating machinery.
Caution should be exercised when prescribing Pregabalin to patients who have had a previous episode of angioedema. In addition, patients who are taking other drugs associated with angioedema (e.g., angiotensin converting enzyme inhibitors [ACE-inhibitors]) may be at increased risk of developing angioedema.
Hypersensitivity: There have been postmarketing reports of hypersensitivity in patients shortly after initiation of treatment with pregabalin. Adverse reactions included skin redness, blisters, hives, rash, dyspnea, and wheezing. Pregabalin should be discontinued immediately in patients with these symptoms.
Withdrawal of Antiepileptic Drugs (AEDs): As with all AEDs, pregabalin should be withdrawn gradually to minimize the potential of increased seizure frequency in patients with seizure disorders. If pregabalin is discontinued this should be done gradually over a minimum of 1 week.
Pregabalin treatment may cause peripheral edema. In short-term trials of patients without clinically significant heart or peripheral vascular disease, there was no apparent association between peripheral edema and cardiovascular complications such as hypertension or congestive heart failure. Peripheral edema was not associated with laboratory changes suggestive of deterioration in renal or hepatic function.
As the thiazolidinedione class of antidiabetic drugs can cause weight gain and/or fluid retention, possibly exacerbating or leading to heart failure, care should be taken when co-administering VEXER and these agents.
Dizziness and Somnolence: VEXER may cause dizziness and somnolence. Patients should be informed that pregabalin-related dizziness and somnolence may impair their ability to perform tasks such as driving or operating machinery.
Special Precautions
Discontinue immediately if angioedema & hypersensitivity occurs. Patients w/ previous episode of angioedema & those taking other drugs associated w/ angioedema (eg, ACE inhibitors). W/draw gradually over a min of 1 wk. Peripheral edema. Co-administration w/ thiazolidinediones. May impair ability to perform tasks eg, driving or operating machinery. Childn <12 yr & adolescents (12-17 yr). Elderly ≥65 yr.
Adverse Reactions
The most commonly reported adverse reactions were dizziness and somnolence. Adverse reactions were usually mild to moderate in intensity.
The adverse reactions listed may also be associated with the underlying disease and/or concomitant medicinal products.
Additional reactions reported from post-marketing experience are included as Frequency not known in italics in the list as follows. (See Tables 2a and 2b.)
The adverse reactions listed may also be associated with the underlying disease and/or concomitant medicinal products.
Additional reactions reported from post-marketing experience are included as Frequency not known in italics in the list as follows. (See Tables 2a and 2b.)
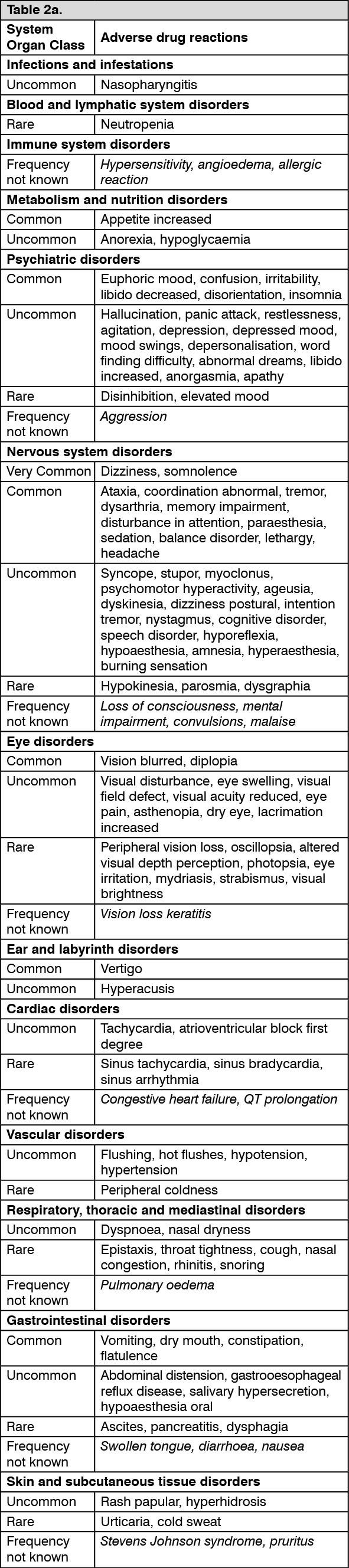

After discontinuation of short-term and long-term treatment with pregabalin withdrawal symptoms have been observed in some patients. The following reactions have been mentioned: insomnia, headache, nausea, anxiety, diarrhoea, flu syndrome, convulsions, nervousness, depression, pain, hyperhidrosis and dizziness. The patient should be informed about this at the start of the treatment.
Concerning discontinuation of long-term treatment of pregabalin there are no data of the incidence and severity of withdrawal symptoms in relation to duration of use and dose of pregabalin.
Concerning discontinuation of long-term treatment of pregabalin there are no data of the incidence and severity of withdrawal symptoms in relation to duration of use and dose of pregabalin.
Drug Interactions
Alcohol and drugs that cause sedation may increase the sedative effects of pregabalin. Pioglitazone (Actos) and rosiglitazone (Avandia) cause weight gain, fluid retention and possibly heart failure. Therefore, combining pregabalin with these drugs may increase the occurrence of weight gain and fluid retention.
Storage
Store at temperatures not exceeding 30ºC. Protect from light.
Shelf-Life: Vexer 50 mg: 36 months from the Date of Manufacturing.
Vexer 75 mg and Vexer 150 mg: 24 months from the Date of Manufacturing.
Shelf-Life: Vexer 50 mg: 36 months from the Date of Manufacturing.
Vexer 75 mg and Vexer 150 mg: 24 months from the Date of Manufacturing.
Action
Pharmacological Classification: Anti-epileptic.
Pharmacology: Pharmacodynamics: Pregabalin binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. Although the mechanism of action of pregabalin has not been fully elucidated, results with genetically modified mice and with compounds structurally related to pregabalin (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin anti-nociceptive and anti-seizure effects in animals. In animal models of nerve damage, pregabalin has been shown to reduce calcium-dependent release of pro-nociceptive neurotransmitters in the spinal cord, possibly by disrupting alpha2-delta containing-calcium channel trafficking and/or reducing calcium currents. Evidence from other animal models of nerve damage and persistent pain suggest the anti-nociceptive activities of pregabalin may also be mediated through interactions with descending noradrenergic and serotonergic pathways originating from the brainstem that modulate pain transmission in the spinal cord. While pregabalin is a structural derivative of the inhibitory neurotransmitter gamma aminobutyric acid (GABA), it does not bind directly to GABAA, GABAB, or benzodiazepine receptors, does not augment GABAA responses in cultured neurons, does not alter rat brain GABA concentration or have acute effects on GABA uptake or degradation. However, in cultured neurons prolonged application of pregabalin increases the density of GABA transporter protein and increases the rate of functional GABA transport. Pregabalin does not block sodium channels, is not active at opiate receptors, and does not alter cyclooxygenase enzyme activity. It is inactive at serotonin and dopamine receptors and does not inhibit dopamine, serotonin, or noradrenaline reuptake.
Pharmacokinetics: Pregabalin is well absorbed after oral administration, is eliminated largely by renal excretion, and has an elimination half-life of about 6 hours.
Absorption and Distribution: Following oral administration of VEXER capsules under fasting conditions, peak plasma concentrations occur within 1.5 hours. Pregabalin oral bioavailability is ≥90% and is independent of dose. Following single- (25 to 300 mg) and multiple-dose (75 to 900 mg/day) administration, maximum plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC) values increase linearly. Following repeated administration, steady state is achieved within 24 to 48 hours. Multiple-dose pharmacokinetics can be predicted from single-dose data.
The rate of pregabalin absorption is decreased when given with food, resulting in a decrease in Cmax of approximately 25% to 30% and an increase in Tmax to approximately 3 hours. However, administration of pregabalin with food has no clinically relevant effect on the total absorption of pregabalin. Therefore, pregabalin can be taken with or without food.
Pregabalin does not bind to plasma proteins. The apparent volume of distribution of pregabalin following oral administration is approximately 0.5 L/kg. Pregabalin is a substrate for system L transporter which is responsible for the transport of large amino acids across the blood brain barrier. Although there are no data in humans, pregabalin has been shown to cross the blood brain barrier in mice, rats, and monkeys. In addition, pregabalin has been shown to cross the placenta in rats and is present in the milk of lactating rats.
Metabolism and Elimination: Pregabalin undergoes negligible metabolism in humans. Following a dose of radiolabeled pregabalin, approximately 90% of the administered dose was recovered in the urine as unchanged pregabalin. The N-methylated derivative of pregabalin, the major metabolite of pregabalin found in urine, accounted for 0.9% of the dose. In preclinical studies, pregabalin (Senantiomer) did not undergo racemization to the R-enantiomer in mice, rats, rabbits, or monkeys.
Pregabalin is eliminated from the systemic circulation primarily by renal excretion as unchanged drug with a mean elimination half-life of 6.3 hours in subjects with normal renal function. Mean renal clearance was estimated to be 67.0 to 80.9 mL/min in young healthy subjects. Because pregabalin is not bound to plasma proteins this clearance rate indicates that renal tubular reabsorption is involved. Pregabalin elimination is nearly proportional to creatinine clearance (Clcr).
Pharmacology: Pharmacodynamics: Pregabalin binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. Although the mechanism of action of pregabalin has not been fully elucidated, results with genetically modified mice and with compounds structurally related to pregabalin (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin anti-nociceptive and anti-seizure effects in animals. In animal models of nerve damage, pregabalin has been shown to reduce calcium-dependent release of pro-nociceptive neurotransmitters in the spinal cord, possibly by disrupting alpha2-delta containing-calcium channel trafficking and/or reducing calcium currents. Evidence from other animal models of nerve damage and persistent pain suggest the anti-nociceptive activities of pregabalin may also be mediated through interactions with descending noradrenergic and serotonergic pathways originating from the brainstem that modulate pain transmission in the spinal cord. While pregabalin is a structural derivative of the inhibitory neurotransmitter gamma aminobutyric acid (GABA), it does not bind directly to GABAA, GABAB, or benzodiazepine receptors, does not augment GABAA responses in cultured neurons, does not alter rat brain GABA concentration or have acute effects on GABA uptake or degradation. However, in cultured neurons prolonged application of pregabalin increases the density of GABA transporter protein and increases the rate of functional GABA transport. Pregabalin does not block sodium channels, is not active at opiate receptors, and does not alter cyclooxygenase enzyme activity. It is inactive at serotonin and dopamine receptors and does not inhibit dopamine, serotonin, or noradrenaline reuptake.
Pharmacokinetics: Pregabalin is well absorbed after oral administration, is eliminated largely by renal excretion, and has an elimination half-life of about 6 hours.
Absorption and Distribution: Following oral administration of VEXER capsules under fasting conditions, peak plasma concentrations occur within 1.5 hours. Pregabalin oral bioavailability is ≥90% and is independent of dose. Following single- (25 to 300 mg) and multiple-dose (75 to 900 mg/day) administration, maximum plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC) values increase linearly. Following repeated administration, steady state is achieved within 24 to 48 hours. Multiple-dose pharmacokinetics can be predicted from single-dose data.
The rate of pregabalin absorption is decreased when given with food, resulting in a decrease in Cmax of approximately 25% to 30% and an increase in Tmax to approximately 3 hours. However, administration of pregabalin with food has no clinically relevant effect on the total absorption of pregabalin. Therefore, pregabalin can be taken with or without food.
Pregabalin does not bind to plasma proteins. The apparent volume of distribution of pregabalin following oral administration is approximately 0.5 L/kg. Pregabalin is a substrate for system L transporter which is responsible for the transport of large amino acids across the blood brain barrier. Although there are no data in humans, pregabalin has been shown to cross the blood brain barrier in mice, rats, and monkeys. In addition, pregabalin has been shown to cross the placenta in rats and is present in the milk of lactating rats.
Metabolism and Elimination: Pregabalin undergoes negligible metabolism in humans. Following a dose of radiolabeled pregabalin, approximately 90% of the administered dose was recovered in the urine as unchanged pregabalin. The N-methylated derivative of pregabalin, the major metabolite of pregabalin found in urine, accounted for 0.9% of the dose. In preclinical studies, pregabalin (Senantiomer) did not undergo racemization to the R-enantiomer in mice, rats, rabbits, or monkeys.
Pregabalin is eliminated from the systemic circulation primarily by renal excretion as unchanged drug with a mean elimination half-life of 6.3 hours in subjects with normal renal function. Mean renal clearance was estimated to be 67.0 to 80.9 mL/min in young healthy subjects. Because pregabalin is not bound to plasma proteins this clearance rate indicates that renal tubular reabsorption is involved. Pregabalin elimination is nearly proportional to creatinine clearance (Clcr).
MedsGo Class
Anticonvulsants
Features
Dosage
75 mg
Ingredients
- Pregabalin
Packaging
Capsule 20's
Generic Name
Pregabalin
Registration Number
DR-XY43500
Classification
Prescription Drug (RX)
Product Questions
Questions


