Indications/Uses
Epoetin beta (Recormon) is indicated for: Treatment of symptomatic anemia associated with chronic kidney disease (CKD) in patients on dialysis.
Treatment of symptomatic renal anemia in patients not yet undergoing dialysis.
Prevention of anemia of prematurity in infants with a birth weight of 750 to 1500 g and a gestational age of less than 34 weeks.
Treatment of symptomatic anemia in adult patients with non-myeloid malignancies receiving chemotherapy.
Increasing the yield of autologous blood from patients in a pre-donation program. Its use in this indication must be balanced against the reported increased risk of thromboembolic events. Treatment should only be given to patients with moderate anemia (Hb 10-13 g/dl [6.21-8.07 mmol/L], no iron deficiency) if blood conserving procedures are not available or insufficient when the scheduled major elective surgery requires a large volume of blood (4 or more units of blood for females or 5 or more units for males).
Treatment of symptomatic renal anemia in patients not yet undergoing dialysis.
Prevention of anemia of prematurity in infants with a birth weight of 750 to 1500 g and a gestational age of less than 34 weeks.
Treatment of symptomatic anemia in adult patients with non-myeloid malignancies receiving chemotherapy.
Increasing the yield of autologous blood from patients in a pre-donation program. Its use in this indication must be balanced against the reported increased risk of thromboembolic events. Treatment should only be given to patients with moderate anemia (Hb 10-13 g/dl [6.21-8.07 mmol/L], no iron deficiency) if blood conserving procedures are not available or insufficient when the scheduled major elective surgery requires a large volume of blood (4 or more units of blood for females or 5 or more units for males).
Dosage/Direction for Use
Therapy with Epoetin beta (Recormon) should be initiated by physicians experienced in the previously mentioned indications. As anaphylactoid reactions were observed in isolated cases, it is recommended that the first dose be administered under medical supervision.
Substitution by any other biological medicinal product requires the consent of the prescribing physician.
Solution for injection in pre-filled syringe: The Epoetin beta (Recormon) pre-filled syringe is ready for use. Under no circumstances should more than one dose be administered per syringe; the medicinal product is for single use only (see Special Instructions for Use, Handling and Disposal under Cautions for Usage).
Treatment of patients with anemia due to chronic kidney disease: The solution can be administered subcutaneously or intravenously. In case of intravenous administration, the solution should be injected over approx. 2 minutes, e.g. in hemodialysis patients via the arteriovenous fistula at the end of dialysis.
For non-hemodialysis patients, subcutaneous administration should always be preferred in order to avoid puncture of peripheral veins.
In CKD patients, the aim of treatment is to reach a target Hb level of 10-12 g/dl. An Hb level of 12 g/dl should not be exceeded. If the rise in hemoglobin is greater than 2 g/dl (1.3 mmol/L) in 4 weeks, an appropriate dose reduction should be considered. In the presence of hypertension or existing cardiovascular, cerebrovascular or peripheral vascular diseases, the weekly increase in Hb and the target Hb should be determined individually taking into account the clinical picture. Patients should be monitored closely to ensure that the lowest dose of Epoetin beta (Recormon) is used to provide adequate control of the symptoms of anemia. Treatment with Epoetin beta (Recormon) is divided into two stages.
1. Correction phase: Subcutaneous administration: The initial dosage is 3 x 20 IU/kg body weight per week. The dosage may be increased every 4 weeks by 3 x 20 IU/kg per week if the increase of Hb is not adequate (< 0.25 g/dl per week).
The weekly dose can also be divided into daily doses.
Intravenous administration: The initial dosage is 3 x 40 IU/kg per week. The dosage may be raised after 4 weeks to 80 IU/kg - three times per week - and by further increments of 20 IU/kg if needed, three times per week, at monthly intervals.
For both routes of administration, the maximum dose should not exceed 720 IU/kg per week.
2. Maintenance phase: To maintain a target Hb value of approximately 10-12 g/dl, the dosage is initially reduced to half of the previously administered amount. Subsequently, the dose is adjusted at intervals of two to four weeks individually for the patient (maintenance dose). In the case of subcutaneous administration, the weekly dose can be given as one injection per week or in divided doses three or seven times per week. Patients who are stable on a once weekly dosing regimen may be switched to once every two weeks administration. In this case, dose increases may be necessary.
Treatment with Epoetin beta (Recormon) is normally a long-term therapy. It can, however, be interrupted, if necessary, at any time. Data on the once weekly dosing schedule are based on clinical studies with a treatment duration of 24 weeks.
Treatment of symptomatic anemia in cancer patients receiving chemotherapy: The solution is administered subcutaneously; the weekly dose can be given as one injection per week or in divided doses 3 to 7 times per week.
The recommended initial dose is 30,000 IU per week (corresponding to approximately 450 IU/kg body weight per week, based on an average weighted patient).
Epoetin beta (Recormon) treatment is indicated if the hemoglobin value is ≤11 g/dl (6.83 mmol/L). Hemoglobin levels should not exceed 13 g/dl (8.07 mmol/L) (see Pharmacology: Pharmacodynamics: Clinical/Efficacy Studies under Actions).
If, after 4 weeks of therapy, the hemoglobin value has increased by at least 1 g/dl (0.62 mmol/L), the current dose should be continued. If the hemoglobin value has not increased by at least 1 g/dl (0.62 mmol/L), a doubling of the weekly dose should be considered. If, after 8 weeks of therapy, the hemoglobin value has not increased by at least 1 g/dl (0.62 mmol/L), response is unlikely and treatment should be discontinued.
The therapy should be continued for up to 4 weeks after the end of chemotherapy.
The maximum dose should not exceed 60,000 IU per week.
Once the therapeutic objective for an individual patient has been achieved, the dose should be reduced by 25 to 50% in order to maintain hemoglobin at that level. If required, further dose reduction may be instituted to ensure that hemoglobin level does not exceed 13 g/dl.
If the rise in hemoglobin is greater than 2 g/dl (1.3 mmol/L) in 4 weeks, the dose should be reduced by 25 to 50%.
Treatment for increasing the amount of autologous blood: The solution is administered intravenously over approx. 2 minutes or subcutaneously. Epoetin beta (Recormon) is administered twice weekly over 4 weeks. On those occasions where the patient's PCV allows blood donation, i.e. PCV ≥33%, Epoetin beta (Recormon) is administered at the end of blood donation.
During the entire treatment period, a PCV of 48% should not be exceeded.
The dosage must be determined by the surgical team individually for each patient as a function of the required amount of pre-donated blood and the endogenous red cell reserve: 1. The required amount of pre-donated blood depends on the anticipated blood loss, use of blood conserving procedures and the physical condition of the patient.
This amount should be that quantity which is expected to be sufficient to avoid homologous blood transfusions.
The required amount of pre-donated blood is expressed in units whereby one unit in the nomogram is equivalent to 180 ml red cells.
2. The ability to donate blood depends predominantly on the patient's blood volume and baseline PCV. Both variables determine the endogenous red cell reserve, which can be calculated according to the following formula. (See equation.)
Substitution by any other biological medicinal product requires the consent of the prescribing physician.
Solution for injection in pre-filled syringe: The Epoetin beta (Recormon) pre-filled syringe is ready for use. Under no circumstances should more than one dose be administered per syringe; the medicinal product is for single use only (see Special Instructions for Use, Handling and Disposal under Cautions for Usage).
Treatment of patients with anemia due to chronic kidney disease: The solution can be administered subcutaneously or intravenously. In case of intravenous administration, the solution should be injected over approx. 2 minutes, e.g. in hemodialysis patients via the arteriovenous fistula at the end of dialysis.
For non-hemodialysis patients, subcutaneous administration should always be preferred in order to avoid puncture of peripheral veins.
In CKD patients, the aim of treatment is to reach a target Hb level of 10-12 g/dl. An Hb level of 12 g/dl should not be exceeded. If the rise in hemoglobin is greater than 2 g/dl (1.3 mmol/L) in 4 weeks, an appropriate dose reduction should be considered. In the presence of hypertension or existing cardiovascular, cerebrovascular or peripheral vascular diseases, the weekly increase in Hb and the target Hb should be determined individually taking into account the clinical picture. Patients should be monitored closely to ensure that the lowest dose of Epoetin beta (Recormon) is used to provide adequate control of the symptoms of anemia. Treatment with Epoetin beta (Recormon) is divided into two stages.
1. Correction phase: Subcutaneous administration: The initial dosage is 3 x 20 IU/kg body weight per week. The dosage may be increased every 4 weeks by 3 x 20 IU/kg per week if the increase of Hb is not adequate (< 0.25 g/dl per week).
The weekly dose can also be divided into daily doses.
Intravenous administration: The initial dosage is 3 x 40 IU/kg per week. The dosage may be raised after 4 weeks to 80 IU/kg - three times per week - and by further increments of 20 IU/kg if needed, three times per week, at monthly intervals.
For both routes of administration, the maximum dose should not exceed 720 IU/kg per week.
2. Maintenance phase: To maintain a target Hb value of approximately 10-12 g/dl, the dosage is initially reduced to half of the previously administered amount. Subsequently, the dose is adjusted at intervals of two to four weeks individually for the patient (maintenance dose). In the case of subcutaneous administration, the weekly dose can be given as one injection per week or in divided doses three or seven times per week. Patients who are stable on a once weekly dosing regimen may be switched to once every two weeks administration. In this case, dose increases may be necessary.
Treatment with Epoetin beta (Recormon) is normally a long-term therapy. It can, however, be interrupted, if necessary, at any time. Data on the once weekly dosing schedule are based on clinical studies with a treatment duration of 24 weeks.
Treatment of symptomatic anemia in cancer patients receiving chemotherapy: The solution is administered subcutaneously; the weekly dose can be given as one injection per week or in divided doses 3 to 7 times per week.
The recommended initial dose is 30,000 IU per week (corresponding to approximately 450 IU/kg body weight per week, based on an average weighted patient).
Epoetin beta (Recormon) treatment is indicated if the hemoglobin value is ≤11 g/dl (6.83 mmol/L). Hemoglobin levels should not exceed 13 g/dl (8.07 mmol/L) (see Pharmacology: Pharmacodynamics: Clinical/Efficacy Studies under Actions).
If, after 4 weeks of therapy, the hemoglobin value has increased by at least 1 g/dl (0.62 mmol/L), the current dose should be continued. If the hemoglobin value has not increased by at least 1 g/dl (0.62 mmol/L), a doubling of the weekly dose should be considered. If, after 8 weeks of therapy, the hemoglobin value has not increased by at least 1 g/dl (0.62 mmol/L), response is unlikely and treatment should be discontinued.
The therapy should be continued for up to 4 weeks after the end of chemotherapy.
The maximum dose should not exceed 60,000 IU per week.
Once the therapeutic objective for an individual patient has been achieved, the dose should be reduced by 25 to 50% in order to maintain hemoglobin at that level. If required, further dose reduction may be instituted to ensure that hemoglobin level does not exceed 13 g/dl.
If the rise in hemoglobin is greater than 2 g/dl (1.3 mmol/L) in 4 weeks, the dose should be reduced by 25 to 50%.
Treatment for increasing the amount of autologous blood: The solution is administered intravenously over approx. 2 minutes or subcutaneously. Epoetin beta (Recormon) is administered twice weekly over 4 weeks. On those occasions where the patient's PCV allows blood donation, i.e. PCV ≥33%, Epoetin beta (Recormon) is administered at the end of blood donation.
During the entire treatment period, a PCV of 48% should not be exceeded.
The dosage must be determined by the surgical team individually for each patient as a function of the required amount of pre-donated blood and the endogenous red cell reserve: 1. The required amount of pre-donated blood depends on the anticipated blood loss, use of blood conserving procedures and the physical condition of the patient.
This amount should be that quantity which is expected to be sufficient to avoid homologous blood transfusions.
The required amount of pre-donated blood is expressed in units whereby one unit in the nomogram is equivalent to 180 ml red cells.
2. The ability to donate blood depends predominantly on the patient's blood volume and baseline PCV. Both variables determine the endogenous red cell reserve, which can be calculated according to the following formula. (See equation.)

The indication for Epoetin beta (Recormon) treatment and, if given, the single dose should be determined from the required amount of pre-donated blood and the endogenous red cell reserve according to the following graphs. (See figure.)
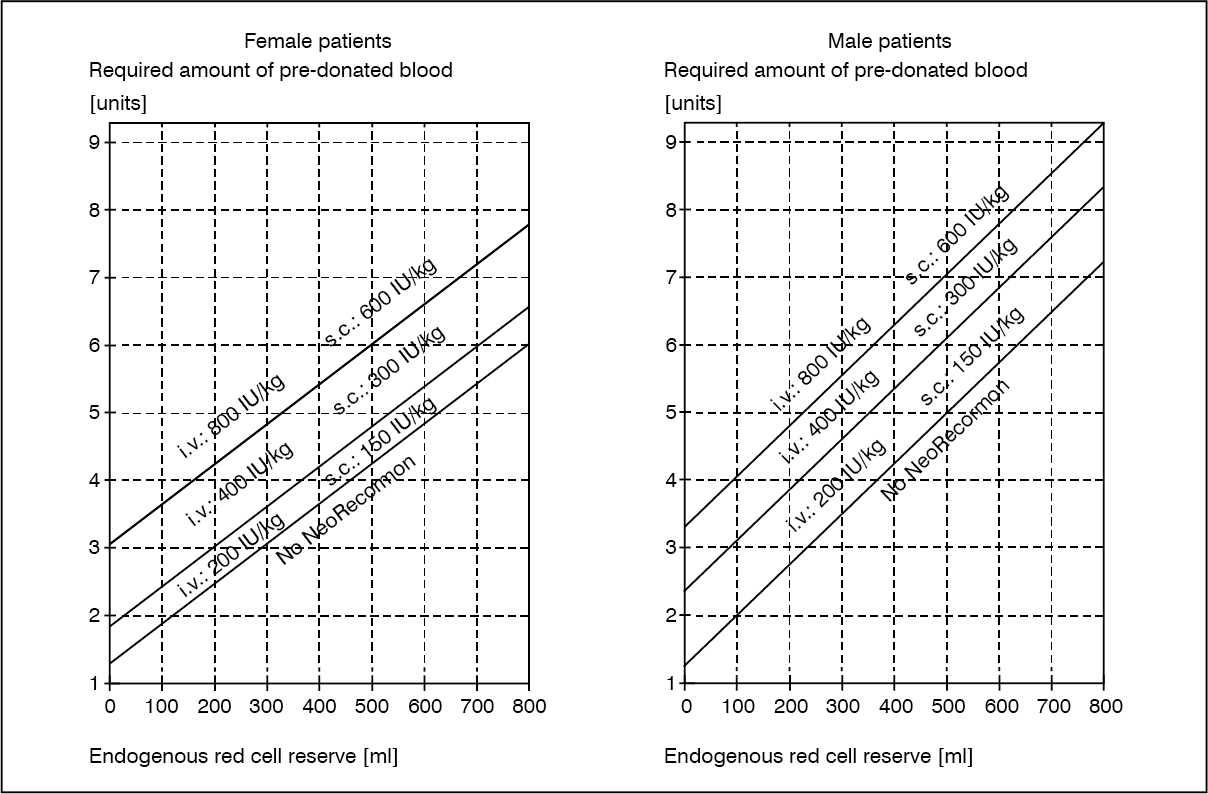
The single dose thus determined is administered twice weekly over 4 weeks. The maximum dose should not exceed 1600 IU/kg body weight per week for intravenous or 1200 IU/kg per week for subcutaneous administration.
Prevention of anemia of prematurity: The solution is administered subcutaneously at a dose of 3 x 250 IU/kg b.w. per week. Epoetin beta (Recormon) treatment should start as early as possible, preferably by day 3 of life. Premature infants who have received a transfusion before starting Epoetin beta (Recormon) treatment are not likely to benefit as much as infants who have not had a transfusion. The treatment should last for 6 weeks.
Special Dosage Instructions: Pediatric use: Results of pediatric clinical studies in have shown that, on average, the younger the patients, the higher the Epoetin beta (Recormon) doses required. Nevertheless, the recommended dosing schedule should be followed as the individual response cannot be predicted (see Use in Children under Precautions).
Geriatric use: No dedicated studies in geriatric patients were performed. A large proportion of geriatric patients were included in clinical trials with Epoetin beta (Recormon). A need for special dose adjustments in the geriatric population was not identified.
Hepatic Impairment: No dedicated clinical trials were conducted in patients with hepatic impairment. No special Dosage Instructions are available.
Prevention of anemia of prematurity: The solution is administered subcutaneously at a dose of 3 x 250 IU/kg b.w. per week. Epoetin beta (Recormon) treatment should start as early as possible, preferably by day 3 of life. Premature infants who have received a transfusion before starting Epoetin beta (Recormon) treatment are not likely to benefit as much as infants who have not had a transfusion. The treatment should last for 6 weeks.
Special Dosage Instructions: Pediatric use: Results of pediatric clinical studies in have shown that, on average, the younger the patients, the higher the Epoetin beta (Recormon) doses required. Nevertheless, the recommended dosing schedule should be followed as the individual response cannot be predicted (see Use in Children under Precautions).
Geriatric use: No dedicated studies in geriatric patients were performed. A large proportion of geriatric patients were included in clinical trials with Epoetin beta (Recormon). A need for special dose adjustments in the geriatric population was not identified.
Hepatic Impairment: No dedicated clinical trials were conducted in patients with hepatic impairment. No special Dosage Instructions are available.
Overdosage
The therapeutic range of Epoetin beta (Recormon) is wide and individual response to therapy must be considered when Epoetin beta (Recormon) treatment is initiated. Overdose can result in manifestations of an exaggerated pharmacodynamic effect, e.g. excessive erythropoiesis which may be associated with life-threatening complications of the cardiovascular system. In case of excessive hemoglobin levels, Epoetin beta (Recormon) should be temporarily withheld (see Dosage & Administration). If clinically indicated, phlebotomy may be performed.
Contraindications
Epoetin beta (Recormon) is contraindicated in patients with: Known hypersensitivity to the active substance or any of the excipients.
Poorly controlled hypertension.
In the indication "increasing the yield of autologous blood", Epoetin beta (Recormon) must not be used in patients who, in the month preceding treatment, have suffered a myocardial infarction or stroke, patients with unstable angina pectoris, or patients who are at increased risk of deep venous thrombosis such as those with a history of venous thromboembolic disease.
Poorly controlled hypertension.
In the indication "increasing the yield of autologous blood", Epoetin beta (Recormon) must not be used in patients who, in the month preceding treatment, have suffered a myocardial infarction or stroke, patients with unstable angina pectoris, or patients who are at increased risk of deep venous thrombosis such as those with a history of venous thromboembolic disease.
Special Precautions
General: In order to improve the traceability of biological medicinal products, the trade name and the batch number of the administered product should be clearly recorded (or stated) in the patient file.
Epoetin beta (Recormon) should be used with caution in the presence of refractory anemia with excess blasts in transformation, epilepsy, thrombocytosis and chronic liver failure. Folic acid and vitamin B12 deficiencies should be ruled out as they reduce the effectiveness of Epoetin beta (Recormon).
In order to ensure effective erythropoiesis, iron status should be evaluated for all patients prior to and during treatment, and supplementary iron therapy may be necessary and conducted in accordance with therapeutic guidelines.
Epoetin beta (Recormon) contains phenylalanine as an excipient. Therefore, this should be taken into consideration in patients affected with severe forms of phenylketonuria.
Lack of effect: The most common reasons for incomplete response to ESAs are iron deficiency and chronic inflammation (e.g. due to uremia or advanced metastatic cancer). The following conditions may also compromise the effectiveness of ESAs therapy: chronic blood loss, bone marrow fibrosis, severe aluminium overload due to treatment of renal failure, folic acid or vitamin B12 deficiencies, and hemolysis. If all the conditions mentioned are excluded and the patient has a sudden drop of hemoglobin associated with reticulocytopenia and anti-erythropoietin antibodies, examination of the bone marrow for the diagnosis of Pure Red Cell Aplasia (PRCA) should be considered. If PRCA is diagnosed, therapy with epoetin beta must be discontinued and patients should not be switched to another ESA.
Pure red cell aplasia caused by neutralising anti-erythropoietin antibodies has been reported in association with erythropoietin therapy, including Epoetin beta (Recormon). These antibodies have been shown to cross-react with all erythropoietic proteins, and patients suspected or confirmed to have neutralising antibodies to erythropoietin should not be switched to Epoetin beta (Recormon) (see Adverse Reactions).
Effect on tumor growth: Epoetins are growth factors that primarily stimulate red blood cell production. Erythropoietin receptors may be expressed on the surface of a variety of tumor cells. As with all growth factors, there is a concern that epoetins could stimulate the growth of any type of malignancy. A controlled clinical study in which epoetin beta was administered to patients with head and neck cancer, has shown a shorter locoregional progression free survival in patients receiving epoetin beta. Another clinical study in breast cancer designed to show a positive effect of epoetin beta on overall survival compared to untreated controls, showed no statistically significant effects in terms of overall survival or tumor progression. Furthermore, meta-analysis data from randomized, controlled clinical studies with epoetin beta in treatment of anemia in cancer patients (12 studies, 2301 patients; including the two studies mentioned previously) did not show any statistically significant negative effects on survival or tumor progression (see Pharmacology: Pharmacodynamics: Clinical/Efficacy Studies under Actions).
In CKD patients and patients with cancer receiving chemotherapy an increase in blood pressure (hypertensive episodes) or aggravation of existing hypertension, especially in cases of rapid Hb increase can occur. Increases in blood pressure can be treated with antihypertensive drugs. If blood pressure rises cannot be controlled by drug therapy, a transient interruption of Epoetin beta (Recormon) therapy is recommended. Particularly at the beginning of therapy, regular monitoring of the blood pressure is recommended, including between dialyses in patients with renal anemia. In patients with CKD, hypertensive crisis with encephalopathy-like symptoms may also occur in individual patients with otherwise normal or low blood pressure. This requires the immediate attention of a physician and intensive medical care. Particular attention should be paid to sudden stabbing migraine-like headaches as a possible warning sign.
Severe aluminium overload due to treatment of renal failure may compromise the effectiveness of Epoetin beta (Recormon).
In CKD patients, an increase in heparin dose during hemodialysis is frequently required during the course of therapy with Epoetin beta (Recormon) as a result of the increased Hb. Occlusion of the dialysis system is possible if heparinization is not optimal. Early shunt revision and thrombosis prophylaxis by administration of acetylsalicylic acid, for example, should be considered in CKD patients at risk of shunt thrombosis.
In CKD patients, there may be a moderate dose-dependent rise in the platelet count within the normal range during treatment with Epoetin beta (Recormon), especially after intravenous administration. This regresses during the course of continued therapy. It is recommended that the platelet count be monitored regularly during the first 8 weeks of therapy.
In patients in an autologous blood predonation program there may be an increase in platelet count, mostly within the normal range. Therefore, it is recommended that the platelet count be determined at least once a week in these patients. If there is an increase in platelets of more than 150 x 109/L or if platelets rise above the normal range, treatment with Epoetin beta (Recormon) should be discontinued.
For use of Epoetin beta (Recormon) in an autologous predonation program, the official guidelines on principles of blood donation must be considered, in particular: only patients with a PCV ≥33% (hemoglobin ≥11 g/dl [6.83 mmol/L]) should donate; special care should be taken with patients below 50 kg weight; the single volume drawn should not exceed approx. 12% of the patient's estimated blood volume.
Treatment should be reserved for patients in whom it is considered of particular importance to avoid homologous blood transfusion taking into consideration the risk/benefit assessment for homologous transfusions.
In patients treated for anemia of prematurity, there may be a slight rise in platelet counts, particularly up to day 12-14 of life, therefore platelets should be monitored regularly.
Laboratory tests: Platelet counts and hematocrit/hemoglobin levels should be monitored at regular intervals in all patients.
In patients with chronic kidney disease, serum potassium elevation has been reported in patients receiving Epoetin beta (Recormon), though causality has not been established. If an elevated or rising potassium level is observed, then consideration should be given to interrupting Epoetin beta (Recormon) administration until the level has been corrected.
Drug Abuse and Dependence: Misuse by non-anemic persons may lead to an excessive increase in Hb. This may be associated with life-threatening complications of the cardiovascular system.
There are no reports on dependence when using epoetin beta.
Renal Impairment: (see General under Precautions).
Hepatic Impairment: (see Special Dosage Instructions under Dosage & Administration).
Ability to Drive and Use Machines: Epoetin beta (Recormon) has no or negligible influence on the ability to drive and use machines.
Use in Children: Clinical registration trials have been performed in children and adolescents with anemia due to chronic kidney disease and in neonates for prevention of anemia due to prematurity.
In the indication anemia due to chronic kidney disease, Epoetin beta (Recormon) should not be used in infants (i.e. below 2 years of age) (see Special Dosage Instructions under Dosage & Administration and General under Precautions).
In the indications anemia in cancer patients receiving chemotherapy and treatment for increasing the amount of autologous blood, Epoetin beta (Recormon) is not indicated in the pediatric population.
Use in the Elderly: (see Special Dosage Instructions under Dosage & Administration).
Epoetin beta (Recormon) should be used with caution in the presence of refractory anemia with excess blasts in transformation, epilepsy, thrombocytosis and chronic liver failure. Folic acid and vitamin B12 deficiencies should be ruled out as they reduce the effectiveness of Epoetin beta (Recormon).
In order to ensure effective erythropoiesis, iron status should be evaluated for all patients prior to and during treatment, and supplementary iron therapy may be necessary and conducted in accordance with therapeutic guidelines.
Epoetin beta (Recormon) contains phenylalanine as an excipient. Therefore, this should be taken into consideration in patients affected with severe forms of phenylketonuria.
Lack of effect: The most common reasons for incomplete response to ESAs are iron deficiency and chronic inflammation (e.g. due to uremia or advanced metastatic cancer). The following conditions may also compromise the effectiveness of ESAs therapy: chronic blood loss, bone marrow fibrosis, severe aluminium overload due to treatment of renal failure, folic acid or vitamin B12 deficiencies, and hemolysis. If all the conditions mentioned are excluded and the patient has a sudden drop of hemoglobin associated with reticulocytopenia and anti-erythropoietin antibodies, examination of the bone marrow for the diagnosis of Pure Red Cell Aplasia (PRCA) should be considered. If PRCA is diagnosed, therapy with epoetin beta must be discontinued and patients should not be switched to another ESA.
Pure red cell aplasia caused by neutralising anti-erythropoietin antibodies has been reported in association with erythropoietin therapy, including Epoetin beta (Recormon). These antibodies have been shown to cross-react with all erythropoietic proteins, and patients suspected or confirmed to have neutralising antibodies to erythropoietin should not be switched to Epoetin beta (Recormon) (see Adverse Reactions).
Effect on tumor growth: Epoetins are growth factors that primarily stimulate red blood cell production. Erythropoietin receptors may be expressed on the surface of a variety of tumor cells. As with all growth factors, there is a concern that epoetins could stimulate the growth of any type of malignancy. A controlled clinical study in which epoetin beta was administered to patients with head and neck cancer, has shown a shorter locoregional progression free survival in patients receiving epoetin beta. Another clinical study in breast cancer designed to show a positive effect of epoetin beta on overall survival compared to untreated controls, showed no statistically significant effects in terms of overall survival or tumor progression. Furthermore, meta-analysis data from randomized, controlled clinical studies with epoetin beta in treatment of anemia in cancer patients (12 studies, 2301 patients; including the two studies mentioned previously) did not show any statistically significant negative effects on survival or tumor progression (see Pharmacology: Pharmacodynamics: Clinical/Efficacy Studies under Actions).
In CKD patients and patients with cancer receiving chemotherapy an increase in blood pressure (hypertensive episodes) or aggravation of existing hypertension, especially in cases of rapid Hb increase can occur. Increases in blood pressure can be treated with antihypertensive drugs. If blood pressure rises cannot be controlled by drug therapy, a transient interruption of Epoetin beta (Recormon) therapy is recommended. Particularly at the beginning of therapy, regular monitoring of the blood pressure is recommended, including between dialyses in patients with renal anemia. In patients with CKD, hypertensive crisis with encephalopathy-like symptoms may also occur in individual patients with otherwise normal or low blood pressure. This requires the immediate attention of a physician and intensive medical care. Particular attention should be paid to sudden stabbing migraine-like headaches as a possible warning sign.
Severe aluminium overload due to treatment of renal failure may compromise the effectiveness of Epoetin beta (Recormon).
In CKD patients, an increase in heparin dose during hemodialysis is frequently required during the course of therapy with Epoetin beta (Recormon) as a result of the increased Hb. Occlusion of the dialysis system is possible if heparinization is not optimal. Early shunt revision and thrombosis prophylaxis by administration of acetylsalicylic acid, for example, should be considered in CKD patients at risk of shunt thrombosis.
In CKD patients, there may be a moderate dose-dependent rise in the platelet count within the normal range during treatment with Epoetin beta (Recormon), especially after intravenous administration. This regresses during the course of continued therapy. It is recommended that the platelet count be monitored regularly during the first 8 weeks of therapy.
In patients in an autologous blood predonation program there may be an increase in platelet count, mostly within the normal range. Therefore, it is recommended that the platelet count be determined at least once a week in these patients. If there is an increase in platelets of more than 150 x 109/L or if platelets rise above the normal range, treatment with Epoetin beta (Recormon) should be discontinued.
For use of Epoetin beta (Recormon) in an autologous predonation program, the official guidelines on principles of blood donation must be considered, in particular: only patients with a PCV ≥33% (hemoglobin ≥11 g/dl [6.83 mmol/L]) should donate; special care should be taken with patients below 50 kg weight; the single volume drawn should not exceed approx. 12% of the patient's estimated blood volume.
Treatment should be reserved for patients in whom it is considered of particular importance to avoid homologous blood transfusion taking into consideration the risk/benefit assessment for homologous transfusions.
In patients treated for anemia of prematurity, there may be a slight rise in platelet counts, particularly up to day 12-14 of life, therefore platelets should be monitored regularly.
Laboratory tests: Platelet counts and hematocrit/hemoglobin levels should be monitored at regular intervals in all patients.
In patients with chronic kidney disease, serum potassium elevation has been reported in patients receiving Epoetin beta (Recormon), though causality has not been established. If an elevated or rising potassium level is observed, then consideration should be given to interrupting Epoetin beta (Recormon) administration until the level has been corrected.
Drug Abuse and Dependence: Misuse by non-anemic persons may lead to an excessive increase in Hb. This may be associated with life-threatening complications of the cardiovascular system.
There are no reports on dependence when using epoetin beta.
Renal Impairment: (see General under Precautions).
Hepatic Impairment: (see Special Dosage Instructions under Dosage & Administration).
Ability to Drive and Use Machines: Epoetin beta (Recormon) has no or negligible influence on the ability to drive and use machines.
Use in Children: Clinical registration trials have been performed in children and adolescents with anemia due to chronic kidney disease and in neonates for prevention of anemia due to prematurity.
In the indication anemia due to chronic kidney disease, Epoetin beta (Recormon) should not be used in infants (i.e. below 2 years of age) (see Special Dosage Instructions under Dosage & Administration and General under Precautions).
In the indications anemia in cancer patients receiving chemotherapy and treatment for increasing the amount of autologous blood, Epoetin beta (Recormon) is not indicated in the pediatric population.
Use in the Elderly: (see Special Dosage Instructions under Dosage & Administration).
Use In Pregnancy & Lactation
Pregnancy: Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/fetal development, parturition or postnatal development (see Pharmacology: Toxicology: Nonclinical Safety under Actions).
For epoetin beta, all safety information with regard to exposure to Epoetin beta (Recormon) during pregnancies has been gained from post marketing experience. A review of the available postmarketing data does not show evidence of a causal association between harmful effects with respect to pregnancy, embryonal/fetal development or postnatal development and treatment with Epoetin beta (Recormon). However in the absence of clinical study data, caution should be exercised when prescribing to pregnant women.
Labor and Delivery: Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/fetal development, parturition or postnatal development (see Pharmacology: Toxicology: Nonclinical Safety under Actions).
For epoetin beta, all safety information with regard to exposure during labor and delivery has been gained from post marketing experience. No evidence of harmful effects with respect to labor and delivery have been observed. However in the absence of clinical study data, caution should be exercised when prescribing to pregnant women in labor.
Lactation: Only limited experience in human lactation has been gained. Endogenous erythropoietin is excreted in breast milk and readily absorbed by the neonatal gastrointestinal tract. A decision on whether to continue or discontinue breast-feeding or to continue or discontinue therapy with epoetin beta should be made taking into account the benefit of breastfeeding to the child and the benefit of epoetin beta therapy to the woman.
For epoetin beta, all safety information with regard to exposure to Epoetin beta (Recormon) during pregnancies has been gained from post marketing experience. A review of the available postmarketing data does not show evidence of a causal association between harmful effects with respect to pregnancy, embryonal/fetal development or postnatal development and treatment with Epoetin beta (Recormon). However in the absence of clinical study data, caution should be exercised when prescribing to pregnant women.
Labor and Delivery: Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/fetal development, parturition or postnatal development (see Pharmacology: Toxicology: Nonclinical Safety under Actions).
For epoetin beta, all safety information with regard to exposure during labor and delivery has been gained from post marketing experience. No evidence of harmful effects with respect to labor and delivery have been observed. However in the absence of clinical study data, caution should be exercised when prescribing to pregnant women in labor.
Lactation: Only limited experience in human lactation has been gained. Endogenous erythropoietin is excreted in breast milk and readily absorbed by the neonatal gastrointestinal tract. A decision on whether to continue or discontinue breast-feeding or to continue or discontinue therapy with epoetin beta should be made taking into account the benefit of breastfeeding to the child and the benefit of epoetin beta therapy to the woman.
Adverse Reactions
Clinical Trials: Based on results from clinical trials including 1725 patients approximately 8% of patients treated with Epoetin beta (Recormon) are expected to experience adverse drug reactions.
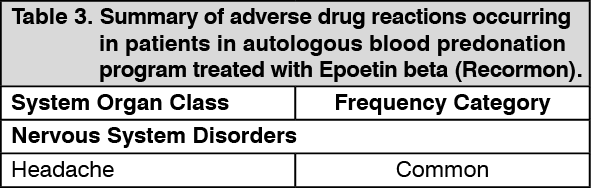
Tabulated summary of adverse drug reactions from clinical trials: Adverse drug reactions from clinical trials (Table 1, Table 2 and Table 3) are listed by MedDRA system organ class. The corresponding frequency category for each adverse drug reaction is based on the following convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1000), very rare (<1/10,000).
Anemic patients due to chronic kidney disease: The most frequent adverse drug reactions (common 1-10%), in particular during the early treatment phase with Epoetin beta (Recormon) are hypertensive events including hypertension, hypertensive crisis with or without encephalopathy-like symptoms (e.g. headaches and confused state, sensorimotor disorders - such as speech disturbance or impaired gait - up to tonoclonic seizures). These increases in blood pressure can occur in normotensive patients or can be an aggravation of existing hypertension (see General under Precautions).
Shunt thromboses may occur, especially in patients who have a tendency to hypotension or whose arteriovenous fistulae exhibit complications (e.g. stenoses, aneurisms) (see General under Precautions). In most cases, a fall in serum ferritin values simultaneous with a rise in Hb is observed. In addition, transient increases in serum potassium and phosphate levels have been observed in isolated cases.
The incidences of adverse drug reactions in clinical trials are shown in the table as follows. The table shows the difference in frequencies of adverse events between patients receiving Epoetin beta (Recormon) and control. (See Table 1.)
Tabulated summary of adverse drug reactions from clinical trials: Adverse drug reactions from clinical trials (Table 1, Table 2 and Table 3) are listed by MedDRA system organ class. The corresponding frequency category for each adverse drug reaction is based on the following convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1000), very rare (<1/10,000).
Anemic patients due to chronic kidney disease: The most frequent adverse drug reactions (common 1-10%), in particular during the early treatment phase with Epoetin beta (Recormon) are hypertensive events including hypertension, hypertensive crisis with or without encephalopathy-like symptoms (e.g. headaches and confused state, sensorimotor disorders - such as speech disturbance or impaired gait - up to tonoclonic seizures). These increases in blood pressure can occur in normotensive patients or can be an aggravation of existing hypertension (see General under Precautions).
Shunt thromboses may occur, especially in patients who have a tendency to hypotension or whose arteriovenous fistulae exhibit complications (e.g. stenoses, aneurisms) (see General under Precautions). In most cases, a fall in serum ferritin values simultaneous with a rise in Hb is observed. In addition, transient increases in serum potassium and phosphate levels have been observed in isolated cases.
The incidences of adverse drug reactions in clinical trials are shown in the table as follows. The table shows the difference in frequencies of adverse events between patients receiving Epoetin beta (Recormon) and control. (See Table 1.)
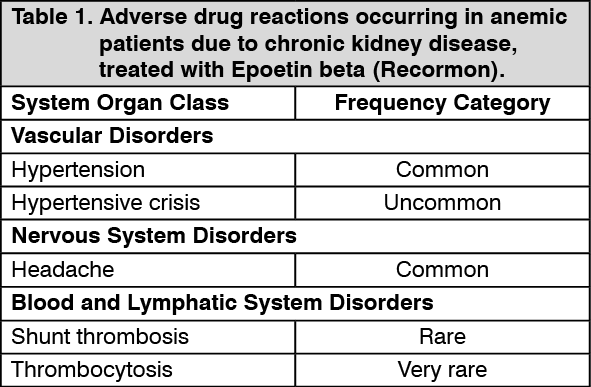
Cancer patients receiving chemotherapy with symptomatic anemia: Hypertensive events are common (1-10%) adverse drug reactions, in particular during the early phase of treatment.
In some patients, a fall in serum iron parameters is observed.
Clinical studies have shown a higher frequency of thromboembolic events in cancer patients treated with Epoetin beta (Recormon) compared to untreated controls or placebo. In patients treated with Epoetin beta (Recormon), this incidence is 7% compared to 4% in controls (both "common"); this is not associated with any increase in thromboembolic mortality compared with controls.
The incidences of adverse drug reactions in clinical trials are shown in the table as follows. The table shows the difference in frequencies of adverse events between patients receiving Epoetin beta (Recormon) and control. (See Table 2.)
In some patients, a fall in serum iron parameters is observed.
Clinical studies have shown a higher frequency of thromboembolic events in cancer patients treated with Epoetin beta (Recormon) compared to untreated controls or placebo. In patients treated with Epoetin beta (Recormon), this incidence is 7% compared to 4% in controls (both "common"); this is not associated with any increase in thromboembolic mortality compared with controls.
The incidences of adverse drug reactions in clinical trials are shown in the table as follows. The table shows the difference in frequencies of adverse events between patients receiving Epoetin beta (Recormon) and control. (See Table 2.)
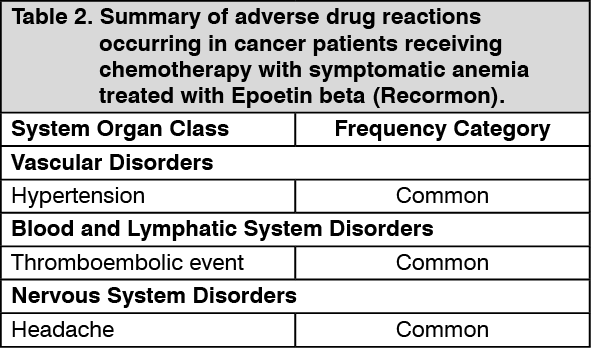
Patients in an autologous blood predonation program: Patients in an autologous blood predonation program have been reported to show a slightly higher frequency of thromboembolic events. However, a causal relationship with treatment with Epoetin beta (Recormon) could not be established.
A temporary iron deficiency may occur (see General under Precautions).
The incidences of adverse drug reactions in clinical trials are shown in the table as follows. The table shows the difference in frequencies of adverse events between patients receiving Epoetin beta (Recormon) and control. (See Table 3.)
A temporary iron deficiency may occur (see General under Precautions).
The incidences of adverse drug reactions in clinical trials are shown in the table as follows. The table shows the difference in frequencies of adverse events between patients receiving Epoetin beta (Recormon) and control. (See Table 3.)
Premature infants: A fall in serum ferritin values is very common (>10%) (see General under Precautions).
All indications: Rarely (≥1/10,000 to ≤1/1,000), skin reactions such as rash, pruritus, urticaria or injection site reactions may occur. In very rare cases (≤1/10,000) anaphylactoid reactions have been reported. However, in controlled clinical studies no increased incidence of hypersensitivity reactions was found.
In very rare cases (≤1/10,000), particularly when starting treatment, flu-like symptoms such as fever, chills, headaches, pain in the limbs, malaise and/or bone pain have been reported. These reactions were mild or moderate in nature and subsided after a couple of hours or days.
Laboratory Abnormalities: (see General under Precautions).
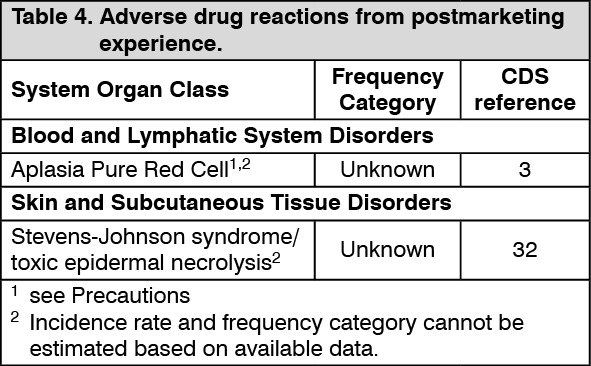
Postmarketing Experience: The following adverse drug reactions have been identified from postmarketing experience with Epoetin beta (Recormon) (Table 4). Adverse drug reactions are listed according to system organ classes in MedDRA and the corresponding frequency category estimation for each adverse drug reaction is based on the following convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1000), very rare (<1/10,000), unknown (cannot be estimated from the available data). (See Table 4.)
All indications: Rarely (≥1/10,000 to ≤1/1,000), skin reactions such as rash, pruritus, urticaria or injection site reactions may occur. In very rare cases (≤1/10,000) anaphylactoid reactions have been reported. However, in controlled clinical studies no increased incidence of hypersensitivity reactions was found.
In very rare cases (≤1/10,000), particularly when starting treatment, flu-like symptoms such as fever, chills, headaches, pain in the limbs, malaise and/or bone pain have been reported. These reactions were mild or moderate in nature and subsided after a couple of hours or days.
Laboratory Abnormalities: (see General under Precautions).
Postmarketing Experience: The following adverse drug reactions have been identified from postmarketing experience with Epoetin beta (Recormon) (Table 4). Adverse drug reactions are listed according to system organ classes in MedDRA and the corresponding frequency category estimation for each adverse drug reaction is based on the following convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1000), very rare (<1/10,000), unknown (cannot be estimated from the available data). (See Table 4.)
Laboratory Abnormalities: Laboratory abnormalities reported during post marketing reflect the experience gained from clinical trials (see General under Precautions and Clinical Trials as previously mentioned).
Caution For Usage
Special Instructions for Use, Handling and Disposal: Incompatibilities: In the absence of compatibility studies, this medicinal product should not be mixed with other medicinal products.
First wash the hands.
1. Remove one syringe from the pack and check that the solution is clear, colorless and practically free from visible particles. Remove the cap from the syringe.
2. Remove one needle from the pack, fix it on the syringe and remove the protective cap from the needle.
3. Expel air from the syringe and needle by holding the syringe vertically and gently pressing the plunger upwards. Keep pressing the plunger until the amount of Epoetin beta (Recormon) in the syringe is as prescribed.
4. Clean the skin at the site of injection using an alcohol wipe. Form a skin fold by pinching the skin between thumb and forefinger. Hold the syringe barrel near to the needle, and insert the needle into the skin fold with a quick, firm action. Inject the Epoetin beta (Recormon) solution. Withdraw the needle quickly and apply pressure over the injection site with a dry, sterile pad.
This medicinal product is for single use only.
Disposal: The following points should be strictly adhered to regarding the use and disposal of syringes and other medicinal sharps: Needles and syringes should never be reused.
Place all used needles and syringes into a sharps container (puncture-proof disposable container).
Keep this container out of the reach of children.
Placing used sharps containers in the household waste should be avoided.
Dispose of the full container according to local requirements or as instructed by the healthcare provider.
The release of pharmaceuticals in the environment should be minimized. Medicines should not be disposed of via wastewater, and disposal through household waste should be avoided. Use established "collection systems" if available in the location.
First wash the hands.
1. Remove one syringe from the pack and check that the solution is clear, colorless and practically free from visible particles. Remove the cap from the syringe.
2. Remove one needle from the pack, fix it on the syringe and remove the protective cap from the needle.
3. Expel air from the syringe and needle by holding the syringe vertically and gently pressing the plunger upwards. Keep pressing the plunger until the amount of Epoetin beta (Recormon) in the syringe is as prescribed.
4. Clean the skin at the site of injection using an alcohol wipe. Form a skin fold by pinching the skin between thumb and forefinger. Hold the syringe barrel near to the needle, and insert the needle into the skin fold with a quick, firm action. Inject the Epoetin beta (Recormon) solution. Withdraw the needle quickly and apply pressure over the injection site with a dry, sterile pad.
This medicinal product is for single use only.
Disposal: The following points should be strictly adhered to regarding the use and disposal of syringes and other medicinal sharps: Needles and syringes should never be reused.
Place all used needles and syringes into a sharps container (puncture-proof disposable container).
Keep this container out of the reach of children.
Placing used sharps containers in the household waste should be avoided.
Dispose of the full container according to local requirements or as instructed by the healthcare provider.
The release of pharmaceuticals in the environment should be minimized. Medicines should not be disposed of via wastewater, and disposal through household waste should be avoided. Use established "collection systems" if available in the location.
Storage
As registered locally.
Store in a refrigerator (2°C-8°C).
Keep the pre-filled syringe in the outer carton, in order to protect from light.
For the purpose of ambulatory use, the patient may remove the pre-filled syringe from the refrigerator and store it at room temperature (not above 25°C) for one single period of up to 3 days.
Shelf life: As registered locally.
Store in a refrigerator (2°C-8°C).
Keep the pre-filled syringe in the outer carton, in order to protect from light.
For the purpose of ambulatory use, the patient may remove the pre-filled syringe from the refrigerator and store it at room temperature (not above 25°C) for one single period of up to 3 days.
Shelf life: As registered locally.
Action
Therapeutic/Pharmacologic Class of Drug: Antianemic agent. ATC code: B03XA.
Pharmacology: The biological efficacy of epoetin beta has been demonstrated after intravenous and subcutaneous administration in various animal models in vivo (normal and uremic rats, polycythemic mice, dogs). After administration of epoetin beta, the number of erythrocytes, the Hb values and reticulocyte counts increase as well as the 59Fe-incorporation rate.
An increased 3H-thymidine incorporation in the erythroid nucleated spleen cells has been found in vitro (mouse spleen cell culture) after incubation with epoetin beta.
Investigations in cell cultures of human bone marrow cells showed that epoetin beta stimulates erythropoiesis specifically and does not affect leucopoiesis. Cytotoxic actions of epoetin beta on bone marrow or on human skin cells were not detected.
After single dose administration of epoetin beta no effects on behavior or locomotor activity of mice and circulatory or respiratory function of dogs were observed.
Pharmacodynamics: Epoetin beta is identical in its amino acid and carbohydrate composition to erythropoietin that has been isolated from the urine of anemic patients.
Erythropoietin is a glycoprotein that stimulates the formation of erythrocytes from its committed progenitors. It acts as a mitosis stimulating factor and differentiation hormone.
Mechanism of Action: Erythropoietin is a glycoprotein that, as a growth factor, primarily stimulates the formation of erythrocytes from its committed progenitors. It acts as a mitosis stimulating factor and differentiation hormone.
Clinical/Efficacy Studies: This section describes recently completed randomized, controlled studies with epoetin beta in patients with renal anemia or cancer patients receiving chemotherapy/radiotherapy.
Patients with anemia due to chronic kidney disease: An open randomized study using epoetin beta was conducted in 605 pre-dialysis patients (CREATE) with mild to moderate anemia (Hb level: 11-12.5 g/dl). The primary objective was to explore whether high Hb correction (13-15 g/dl) would reduce cardiovascular (CV) morbidity as compared with standard anemia treatment (target Hb 10.5-11.5 g/dl). There was no benefit observed with high Hb correction compared to standard anemia correction. On the contrary, there were fewer events observed in the standard treatment group (47 versus 58 events, HR 0.78, p=0.20). A difference in time to initiation of dialysis was observed favoring the standard anemia correction group (111 and 127 events, median time to dialysis 41 months and 36 months, log rank test p=0.034, respectively), although no difference in median creatinine clearance over time between the two study groups was observed. Quality of life (assessed by SF-36 Health Survey Questionnaire) was significantly improved (p=0.003) in the high target Hb group at 1 year.
In another open randomized study in 172 patients with early diabetic nephrology, (ACORD) the effect of high Hb correction (target Hb 13-15 g/dl) and standard Hb correction (target Hb 10.5-11.5 g/dl) on cardiac structure and function was investigated.
At the end of the study, there was no significant difference between the two groups with respect to the primary parameter, the baseline adjusted left ventricular mass index (p=0.88). There was no statistically significant difference between the treatment groups in change from baseline in calculated creatinine clearance, time to doubling of serum creatinine, or an analysis of rapid progressors. The General Health score of the quality of life assessment (using the SF-36 Health Survey Questionnaire) was significantly improved (p=0.04) in the high target Hb group.
Cancer patients with symptomatic anemia receiving chemotherapy: In a placebo-controlled study using epoetin beta in 351 patients with head and neck cancer (ENHANCE), study drug was administered to maintain the hemoglobin levels of 14 g/dl in women and 15 g/dl in men. Locoregional progression free survival was significantly shorter in patients receiving epoetin beta (HR=1.62, p=0.0008). The results and interpretation of this study were confounded by imbalances between the treatment groups, especially with regard to tumor localization, smoking status and the heterogeneity of the study population.
A controlled, open label, randomized study using epoetin beta in 463 patients with metastatic breast cancer receiving chemotherapy (BRAVE), which was designed to show a significant improvement in survival, did not show any statistically significant difference between the control and epoetin beta arms with regards to overall survival (p=0.52) or time to tumor progression (p=0.45). A greater number of patients in the control arm (64/232; 27.6%) had transfusion and severe anemic events compared with the epoetin beta arm (40/231; 17.3%) (p=0.009), reflecting the efficacy of epoetin beta treatment with respect to preventing transfusions by effective increase in hemoglobin.
A higher percentage of epoetin beta patients experienced thromboembolic events (TEEs) during the study compared with the control arm (13% versus 6%) and a shorter time to TEE for the epoetin beta treatment arm compared with control (p=0.008) was seen. However, the percentage of patients that experienced a serious TEE (3% control versus 4% epoetin beta) or TEE leading to death (2% in each arm) was comparable.
A controlled, open label, randomized study using epoetin beta in 74 patients with cervical cancer receiving radiochemotherapy (MARCH) did not show a correlation between hemoglobin increase and the reduction in treatment failures (response to radiochemotherapy). Therefore, it was decided not to proceed this study to its second stage.
A meta-analysis including all controlled clinical studies in anemic cancer patients treated with epoetin beta was performed (12 studies with a total of 2301 patients). The results from this present meta-analysis confirm the known efficacy of epoetin beta with respect to increases in hemoglobin levels and a reduced risk of blood transfusion.
In the overall population including also patients with Hb initiation levels up to 13 g/dl, no statistically significant increase in risk of death in the epoetin beta group compared with the control group (HR: 1.13, 95% CI 0.87 to 1.46, p=0.34) was observed. In patients with baseline hemoglobin ≤ 11 g/dl, the HR for overall survival was 1.09 (95% CI 0.80 to 1.47, p=0.58). For time to disease progression the HR was 0.85 (95% CI: 0.72 to 1.01, p=0.07) in the overall patient population. When the analysis was restricted to patients with baseline hemoglobin ≤ 11 g/dl the HR was 0.80 (95% CI 0.65 to 0.99, p=0.04).
This meta analysis also confirmed the increased rate of thromboembolic events (TEE) reported (see Adverse Reactions) with a TEE rate of 7% in the epoetin beta group compared with 4% in the control group.
Immunogenicity: (see General under Precautions).
Pharmacokinetics: Pharmacokinetic investigations in healthy volunteers and uremic patients show that the half-life of intravenously administered epoetin beta is between 4 and 12 hours and that the distribution volume corresponds to one to two times the plasma volume. Analogous results have been found in animal experiments in uremic and normal rats.
Absorption: After subcutaneous administration of epoetin beta to uremic patients, the protracted absorption results in a serum concentration plateau, whereby the maximum concentration is reached after an average of 12-28 hours.
Bioavailability of epoetin beta after subcutaneous administration is between 23 and 42% as compared with intravenous administration.
Distribution: Pharmacokinetic investigations in healthy volunteers and uremic patients show that the distribution volume corresponds to one to two times the plasma volume.
Metabolism: Not applicable.
Elimination: Pharmacokinetic investigations in healthy volunteers and uremic patients show that the half-life of intravenously administered epoetin beta is between 4 and 12 hours.
After subcutaneous administration of epoetin beta to uremic patients, the terminal half-life is higher than after intravenous administration, with an average of 13-28 hours.
Pharmacokinetics in Special Populations: No formal study of the effect of hepatic impairment on the pharmacokinetics of epoetin beta was conducted.
Toxicology: Nonclinical Safety: Carcinogenicity: A carcinogenicity study with homologous erythropoietin in mice did not reveal any signs of proliferative or tumorigenic potential.
Genotoxicity: Not applicable.
Impairment of Fertility: Not applicable.
Reproductive toxicity: Not applicable.
Other: Nonclinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, and toxicity of reproduction.
Pharmacology: The biological efficacy of epoetin beta has been demonstrated after intravenous and subcutaneous administration in various animal models in vivo (normal and uremic rats, polycythemic mice, dogs). After administration of epoetin beta, the number of erythrocytes, the Hb values and reticulocyte counts increase as well as the 59Fe-incorporation rate.
An increased 3H-thymidine incorporation in the erythroid nucleated spleen cells has been found in vitro (mouse spleen cell culture) after incubation with epoetin beta.
Investigations in cell cultures of human bone marrow cells showed that epoetin beta stimulates erythropoiesis specifically and does not affect leucopoiesis. Cytotoxic actions of epoetin beta on bone marrow or on human skin cells were not detected.
After single dose administration of epoetin beta no effects on behavior or locomotor activity of mice and circulatory or respiratory function of dogs were observed.
Pharmacodynamics: Epoetin beta is identical in its amino acid and carbohydrate composition to erythropoietin that has been isolated from the urine of anemic patients.
Erythropoietin is a glycoprotein that stimulates the formation of erythrocytes from its committed progenitors. It acts as a mitosis stimulating factor and differentiation hormone.
Mechanism of Action: Erythropoietin is a glycoprotein that, as a growth factor, primarily stimulates the formation of erythrocytes from its committed progenitors. It acts as a mitosis stimulating factor and differentiation hormone.
Clinical/Efficacy Studies: This section describes recently completed randomized, controlled studies with epoetin beta in patients with renal anemia or cancer patients receiving chemotherapy/radiotherapy.
Patients with anemia due to chronic kidney disease: An open randomized study using epoetin beta was conducted in 605 pre-dialysis patients (CREATE) with mild to moderate anemia (Hb level: 11-12.5 g/dl). The primary objective was to explore whether high Hb correction (13-15 g/dl) would reduce cardiovascular (CV) morbidity as compared with standard anemia treatment (target Hb 10.5-11.5 g/dl). There was no benefit observed with high Hb correction compared to standard anemia correction. On the contrary, there were fewer events observed in the standard treatment group (47 versus 58 events, HR 0.78, p=0.20). A difference in time to initiation of dialysis was observed favoring the standard anemia correction group (111 and 127 events, median time to dialysis 41 months and 36 months, log rank test p=0.034, respectively), although no difference in median creatinine clearance over time between the two study groups was observed. Quality of life (assessed by SF-36 Health Survey Questionnaire) was significantly improved (p=0.003) in the high target Hb group at 1 year.
In another open randomized study in 172 patients with early diabetic nephrology, (ACORD) the effect of high Hb correction (target Hb 13-15 g/dl) and standard Hb correction (target Hb 10.5-11.5 g/dl) on cardiac structure and function was investigated.
At the end of the study, there was no significant difference between the two groups with respect to the primary parameter, the baseline adjusted left ventricular mass index (p=0.88). There was no statistically significant difference between the treatment groups in change from baseline in calculated creatinine clearance, time to doubling of serum creatinine, or an analysis of rapid progressors. The General Health score of the quality of life assessment (using the SF-36 Health Survey Questionnaire) was significantly improved (p=0.04) in the high target Hb group.
Cancer patients with symptomatic anemia receiving chemotherapy: In a placebo-controlled study using epoetin beta in 351 patients with head and neck cancer (ENHANCE), study drug was administered to maintain the hemoglobin levels of 14 g/dl in women and 15 g/dl in men. Locoregional progression free survival was significantly shorter in patients receiving epoetin beta (HR=1.62, p=0.0008). The results and interpretation of this study were confounded by imbalances between the treatment groups, especially with regard to tumor localization, smoking status and the heterogeneity of the study population.
A controlled, open label, randomized study using epoetin beta in 463 patients with metastatic breast cancer receiving chemotherapy (BRAVE), which was designed to show a significant improvement in survival, did not show any statistically significant difference between the control and epoetin beta arms with regards to overall survival (p=0.52) or time to tumor progression (p=0.45). A greater number of patients in the control arm (64/232; 27.6%) had transfusion and severe anemic events compared with the epoetin beta arm (40/231; 17.3%) (p=0.009), reflecting the efficacy of epoetin beta treatment with respect to preventing transfusions by effective increase in hemoglobin.
A higher percentage of epoetin beta patients experienced thromboembolic events (TEEs) during the study compared with the control arm (13% versus 6%) and a shorter time to TEE for the epoetin beta treatment arm compared with control (p=0.008) was seen. However, the percentage of patients that experienced a serious TEE (3% control versus 4% epoetin beta) or TEE leading to death (2% in each arm) was comparable.
A controlled, open label, randomized study using epoetin beta in 74 patients with cervical cancer receiving radiochemotherapy (MARCH) did not show a correlation between hemoglobin increase and the reduction in treatment failures (response to radiochemotherapy). Therefore, it was decided not to proceed this study to its second stage.
A meta-analysis including all controlled clinical studies in anemic cancer patients treated with epoetin beta was performed (12 studies with a total of 2301 patients). The results from this present meta-analysis confirm the known efficacy of epoetin beta with respect to increases in hemoglobin levels and a reduced risk of blood transfusion.
In the overall population including also patients with Hb initiation levels up to 13 g/dl, no statistically significant increase in risk of death in the epoetin beta group compared with the control group (HR: 1.13, 95% CI 0.87 to 1.46, p=0.34) was observed. In patients with baseline hemoglobin ≤ 11 g/dl, the HR for overall survival was 1.09 (95% CI 0.80 to 1.47, p=0.58). For time to disease progression the HR was 0.85 (95% CI: 0.72 to 1.01, p=0.07) in the overall patient population. When the analysis was restricted to patients with baseline hemoglobin ≤ 11 g/dl the HR was 0.80 (95% CI 0.65 to 0.99, p=0.04).
This meta analysis also confirmed the increased rate of thromboembolic events (TEE) reported (see Adverse Reactions) with a TEE rate of 7% in the epoetin beta group compared with 4% in the control group.
Immunogenicity: (see General under Precautions).
Pharmacokinetics: Pharmacokinetic investigations in healthy volunteers and uremic patients show that the half-life of intravenously administered epoetin beta is between 4 and 12 hours and that the distribution volume corresponds to one to two times the plasma volume. Analogous results have been found in animal experiments in uremic and normal rats.
Absorption: After subcutaneous administration of epoetin beta to uremic patients, the protracted absorption results in a serum concentration plateau, whereby the maximum concentration is reached after an average of 12-28 hours.
Bioavailability of epoetin beta after subcutaneous administration is between 23 and 42% as compared with intravenous administration.
Distribution: Pharmacokinetic investigations in healthy volunteers and uremic patients show that the distribution volume corresponds to one to two times the plasma volume.
Metabolism: Not applicable.
Elimination: Pharmacokinetic investigations in healthy volunteers and uremic patients show that the half-life of intravenously administered epoetin beta is between 4 and 12 hours.
After subcutaneous administration of epoetin beta to uremic patients, the terminal half-life is higher than after intravenous administration, with an average of 13-28 hours.
Pharmacokinetics in Special Populations: No formal study of the effect of hepatic impairment on the pharmacokinetics of epoetin beta was conducted.
Toxicology: Nonclinical Safety: Carcinogenicity: A carcinogenicity study with homologous erythropoietin in mice did not reveal any signs of proliferative or tumorigenic potential.
Genotoxicity: Not applicable.
Impairment of Fertility: Not applicable.
Reproductive toxicity: Not applicable.
Other: Nonclinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, and toxicity of reproduction.
MedsGo Class
Haematopoietic Agents
Features
Dosage
5000 IU / 0.3 ml
Ingredients
- Epoetin Beta
Packaging
Solution for Injection (I.V./S.C.) 0.3ml x 1's
Generic Name
Epoetin Beta
Registration Number
BR-1061
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions


