All variations
Indications/Uses
Indicated bacteria: Susceptible strains of Staphylococcus sp., Streptococcus pneumoniae, Enterococcus sp., Micrococcus sp., Moraxella sp., Corynebacterium sp., Klebsiella sp., Enterobacter sp., Serratia sp., Proteus sp., Morganella morganii, Haemophilus influenzae, Haemophilus aegyptius [Koch-Weeks bacillus], Pseudomonas sp., Pseudomonas aeruginosa, Stenotrophomonas (Xanthomonas) maltophilia, Acinetobacter sp., and Propionibacterium acnes.
Indications: Blepharitis, dacryocystitis, hordeolum, conjunctivitis, tarsadenitis, keratitis (including corneal ulcer), and aseptic treatment during perioperative period for ocular surgery.
Indications: Blepharitis, dacryocystitis, hordeolum, conjunctivitis, tarsadenitis, keratitis (including corneal ulcer), and aseptic treatment during perioperative period for ocular surgery.
Dosage/Direction for Use
Usually, instill 1 drop a time to the eye 3 times daily.
The dosage may be adjusted according to the patient's symptoms.
The dosage may be adjusted according to the patient's symptoms.
Contraindications
(Oftaquix ophthalmic solution is contraindicated in the following patients.)
Patients with history of hypersensitivity to the ingredient of this product, ofloxacin or any quinolone antibiotics.
Patients with history of hypersensitivity to the ingredient of this product, ofloxacin or any quinolone antibiotics.
Special Precautions
Precautions concerning use: Route of administration: Ophthalmic use only.
At the time of administration: Instruct the patient to be careful not to touch the tip of the bottle to the eye directly in order to avoid the contamination of the drug. When more than one ophthalmic drug is used, at least 5 minutes of intervals should be taken.
Use in Pregnancy & Lactation: This product should be used in pregnant women or women who may possibly be pregnant only if the expected therapeutic benefits are judged to outweigh the possible risk associated with treatment. (The safety of this product during pregnancy has not been established.)
At the time of administration: Instruct the patient to be careful not to touch the tip of the bottle to the eye directly in order to avoid the contamination of the drug. When more than one ophthalmic drug is used, at least 5 minutes of intervals should be taken.
Use in Pregnancy & Lactation: This product should be used in pregnant women or women who may possibly be pregnant only if the expected therapeutic benefits are judged to outweigh the possible risk associated with treatment. (The safety of this product during pregnancy has not been established.)
Use In Pregnancy & Lactation
This product should be used in pregnant women or women who may possibly be pregnant only if the expected therapeutic benefits are judged to outweigh the possible risk associated with treatment. (The safety of this product during pregnancy has not been established.)
Adverse Reactions
Clinically significant adverse reactions: Shock, anaphylactoid reaction: Since shock and anaphylactoid reaction may occur, patients should be carefully observed. If any symptoms such as erythema, rash, dyspnoea, decreased blood pressure, and eyelid oedema, etc. are observed, administration should be discontinued and appropriate measures should be taken.
Other adverse reactions: If the following adverse reactions are observed, appropriate measures such as discontinuing administration should be taken.
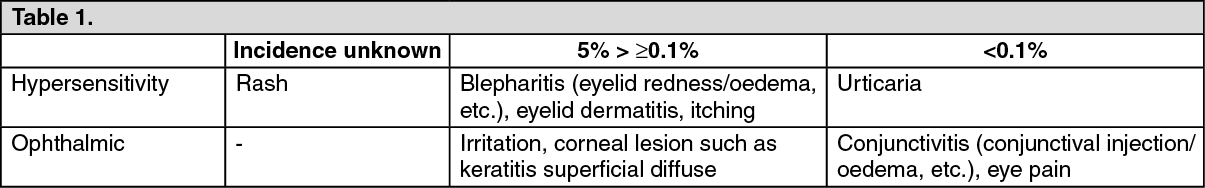
5 mg/mL: See Table 1.
Other adverse reactions: If the following adverse reactions are observed, appropriate measures such as discontinuing administration should be taken.
5 mg/mL: See Table 1.
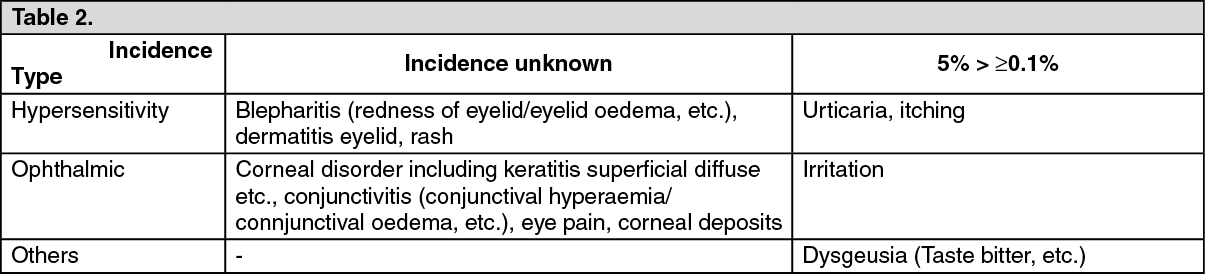
15 mg/mL: See Table 2.
Adverse reactions were reported in 7 of 238 patients (2.9%) in clinical trials in Japan. The adverse reactions were eye irritation in 3 patients (1.3%), dysgeusia in 2 patients (0.8%), eye itching in 1 patient (0.4%), and urticaria in 1 patient (0.4%).
Storage
Store at temperatures not exceeding 30°C. Protect from light.
MedsGo Class
Eye Anti-Infectives & Antiseptics
Features
Dosage
15 mg / ml
Ingredients
- Levofloxacin
Packaging
Ophthalmic Solution 5ml
Generic Name
Levofloxacin Hemihydrate
Registration Number
DR-XY46076
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions




