Indications/Uses
Rifampicin + Isoniazid Fixed-Dose Combination is indicated in the treatment of all forms of tuberculosis, including fresh, advanced and chronic cases.
Dosage/Direction for Use
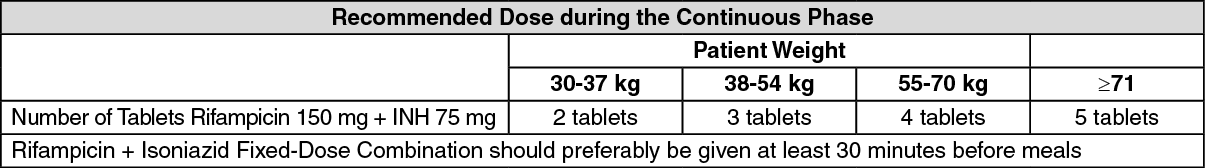
All patients (including those with HIV) infection who have not been treated previously should receive a first-line treatment regimen. The initial phase should consists of two months of Isoniazid + Rifampicin + Pyrazinamide + Ethambutol. The continuation phase should consist of Rifampicin + Isoniazid given for four months. See table.
Overdosage
Signs and Symptoms: Rifampicin: Nausea, vomiting, abdominal pain, pruritus, headache and increasing lethargy will probably occur within a short time after acute ingestion; unconsciousness may occur when there is severe hepatic disease. Transient increases in liver enzymes and/or bilirubin may occur. Brownish-red or orange coloration of the skin, urine, sweat, saliva, tears and feces will occur, and its intensity is proportional to the amount ingested. Facial or periorbital edema has also been reported in pediatric patients. Hypotension, sinus tachycardia, ventricular arrhythmias, seizures and cardiac arrest were reported in some fatal cases.
The minimum acute lethal or toxic dose is not well established. However, nonfatal acute overdoses in adults have been reported with doses ranging from 9 to 12 g rifampicin. Fatal acute overdoses in adults have been reported with doses ranging from 14 to 60 g. Alcohol or a history of alcohol abuse was involved in some of the fatal and nonfatal reports. Nonfatal overdoses in pediatric patients ages 1 to 4 years old of 100 mg/kg for one to two doses have been reported.
Isoniazid: Isoniazid overdosage produces signs and symptoms within 30 minutes to 3 hours after ingestion. Nausea, vomiting, dizziness, slurring of speech, blurring of vision, and visual hallucinations (including bright colors and strange designs) are among the early manifestations. With marked overdosage, respiratory distress and CNS depression, progressing rapidly from stupor to profound coma are to be expected, along with severe, intractable seizures. Severe metabolic acidosis, acetonuria and hyperglycemia are typical laboratory findings.
Treatment: In cases of overdosage with Rifampicin + Isoniazid Fixed-Dose Combination, gastric lavage should be performed as soon as possible. Following evacuation of the gastric contents, the instillation of activated charcoal slurry into the stomach may help absorb any remaining drug from the gastrointestinal tract. Antiemetic medication may be required to control severe nausea and vomiting.
Intensive supportive measures should be instituted, including airway patency, and individual symptoms treated as they arise.
If acute isoniazid overdose is suspected, even in asymptomatic patients, the administration of intravenous pyridoxine (vitamin B6) should be considered. In patients with seizures not controlled with pyridoxine, anticonvulsant therapy should be administered. Sodium bicarbonate should be given to control metabolic acidosis. Hemodialysis is advised for refractory cases; if this is not available, peritoneal dialysis can be used along with forced diuresis.
The minimum acute lethal or toxic dose is not well established. However, nonfatal acute overdoses in adults have been reported with doses ranging from 9 to 12 g rifampicin. Fatal acute overdoses in adults have been reported with doses ranging from 14 to 60 g. Alcohol or a history of alcohol abuse was involved in some of the fatal and nonfatal reports. Nonfatal overdoses in pediatric patients ages 1 to 4 years old of 100 mg/kg for one to two doses have been reported.
Isoniazid: Isoniazid overdosage produces signs and symptoms within 30 minutes to 3 hours after ingestion. Nausea, vomiting, dizziness, slurring of speech, blurring of vision, and visual hallucinations (including bright colors and strange designs) are among the early manifestations. With marked overdosage, respiratory distress and CNS depression, progressing rapidly from stupor to profound coma are to be expected, along with severe, intractable seizures. Severe metabolic acidosis, acetonuria and hyperglycemia are typical laboratory findings.
Treatment: In cases of overdosage with Rifampicin + Isoniazid Fixed-Dose Combination, gastric lavage should be performed as soon as possible. Following evacuation of the gastric contents, the instillation of activated charcoal slurry into the stomach may help absorb any remaining drug from the gastrointestinal tract. Antiemetic medication may be required to control severe nausea and vomiting.
Intensive supportive measures should be instituted, including airway patency, and individual symptoms treated as they arise.
If acute isoniazid overdose is suspected, even in asymptomatic patients, the administration of intravenous pyridoxine (vitamin B6) should be considered. In patients with seizures not controlled with pyridoxine, anticonvulsant therapy should be administered. Sodium bicarbonate should be given to control metabolic acidosis. Hemodialysis is advised for refractory cases; if this is not available, peritoneal dialysis can be used along with forced diuresis.
Administration
Should be taken on an empty stomach: Take at least 30 min before meals.
Contraindications
Rifampicin + Isoniazid Fixed-Dose Combination is contraindicated in patients who are hypersensitive to rifampicin or isoniazid or any of the excipients. Rifampicin + Isoniazid Fixed-Dose Combination is contraindicated in the presence of jaundice.
Rifampicin + Isoniazid Fixed-Dose Combination use is contraindicated when given concurrently with the combination of saquinavir/ritonavir.
Rifampicin + Isoniazid Fixed-Dose Combination use is contraindicated when given concurrently with the combination of saquinavir/ritonavir.
Special Precautions
There have been reports of severe cutaneous adverse reactions (SCARs), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) with anti-tuberculosis drugs (see Adverse Reactions). If patients develop a skin rash they should be monitored closely and suspect drug(s) discontinued if lesions progress. Identifying the specific drug is difficult, as multiple anti-tuberculosis drugs are prescribed in association concurrently. Specifically, for DRESS, a multi-system potential life-threatening SCAR, time to onset of the first symptoms may be prolonged. DRESS is a clinical diagnosis, and its clinical presentation remains the basis for decision making. An early withdrawal of the suspect drug is essential because of the syndrome's mortality and visceral involvement (e.g., liver, bone marrow or kidney).
Rifampicin + Isoniazid Fixed-Dose Combination is a combination of 2 drugs, each of which has been associated with liver dysfunction.
All tuberculosis patients should have pre-treatment measurements of liver function. Adults treated for tuberculosis with Rifampicin + Isoniazid Fixed-Dose Combination should have baseline measurements of hepatic enzymes, bilirubin, serum creatinine, a complete blood count and a platelet count (or estimate).
Patients should be seen at least monthly during therapy and should be questioned specifically about symptoms associated with adverse reactions.
All patients with abnormalities should have a follow-up, including laboratory testing, if necessary. However, because there is a higher frequency of isoniazid-associated hepatitis among persons older than 35 years of age, a transaminase measurement should be obtained at baseline and at least monthly during therapy in this age group. Other factors associated with an increased risk of hepatitis include daily use of alcohol, chronic liver disease, intravenous drug use and being a Black or Hispanic woman.
If the patient has no evidence of pre-existing liver disease and normal pre-treatment liver function, liver function tests need only be repeated if fever, vomiting, jaundice or other deterioration in the patient's condition occurs.
Rifampicin: Serious immunological reactions resulting in renal impairment, hemolysis or thrombocytopenia are on record in patients who resume taking rifampicin after a prolonged lapse of treatment. In this rare situation, rifampicin should be immediately and permanently withdrawn.
Clinical monitoring (and liver function tests, if possible) should be performed during treatment of all patients with pre-existing liver disease, who are at increased risk of further liver damage.
Patients should be warned that treatment may cause reddish coloration of all body secretions (urine, tears, saliva, sweat, semen and sputum), and that contact lenses and clothing may be irreversibly stained.
Isoniazid: Use of isoniazid should be carefully monitored in patients with current chronic liver disease or severe renal dysfunction.
Severe and sometimes fatal hepatitis associated with isoniazid therapy may occur and may develop even after many months of treatment. The risk of developing hepatitis is age related. Therefore, patients should be monitored for the prodromal symptoms of hepatitis, such as fatigue, weakness, malaise, anorexia, nausea or vomiting. If these symptoms appear or if signs suggestive of hepatic damage are detected, isoniazid should be discontinued promptly, since continued use of the drug in these cases has been reported to cause a more severe form of liver damage.
Care should be exercised in the treatment of elderly or malnourished patients who may also require vitamin B6 supplementation with the isoniazid therapy.
Use of isoniazid should be carefully monitored in patients with slow acetylator status, epilepsy, history of psychosis, history of peripheral neuropathy, diabetes, alcohol dependence, HIV infection or porphyria.
Effects on the Ability to Drive and Use Machines: Isoniazid has been associated with vertigo, visual disorders and psychotic reactions (see Adverse Reactions). Patients should be informed of these, and advised that if affected, they should not drive, operate machinery or take part in any activities where these symptoms may put either themselves or others at risk.
Rifampicin + Isoniazid Fixed-Dose Combination is a combination of 2 drugs, each of which has been associated with liver dysfunction.
All tuberculosis patients should have pre-treatment measurements of liver function. Adults treated for tuberculosis with Rifampicin + Isoniazid Fixed-Dose Combination should have baseline measurements of hepatic enzymes, bilirubin, serum creatinine, a complete blood count and a platelet count (or estimate).
Patients should be seen at least monthly during therapy and should be questioned specifically about symptoms associated with adverse reactions.
All patients with abnormalities should have a follow-up, including laboratory testing, if necessary. However, because there is a higher frequency of isoniazid-associated hepatitis among persons older than 35 years of age, a transaminase measurement should be obtained at baseline and at least monthly during therapy in this age group. Other factors associated with an increased risk of hepatitis include daily use of alcohol, chronic liver disease, intravenous drug use and being a Black or Hispanic woman.
If the patient has no evidence of pre-existing liver disease and normal pre-treatment liver function, liver function tests need only be repeated if fever, vomiting, jaundice or other deterioration in the patient's condition occurs.
Rifampicin: Serious immunological reactions resulting in renal impairment, hemolysis or thrombocytopenia are on record in patients who resume taking rifampicin after a prolonged lapse of treatment. In this rare situation, rifampicin should be immediately and permanently withdrawn.
Clinical monitoring (and liver function tests, if possible) should be performed during treatment of all patients with pre-existing liver disease, who are at increased risk of further liver damage.
Patients should be warned that treatment may cause reddish coloration of all body secretions (urine, tears, saliva, sweat, semen and sputum), and that contact lenses and clothing may be irreversibly stained.
Isoniazid: Use of isoniazid should be carefully monitored in patients with current chronic liver disease or severe renal dysfunction.
Severe and sometimes fatal hepatitis associated with isoniazid therapy may occur and may develop even after many months of treatment. The risk of developing hepatitis is age related. Therefore, patients should be monitored for the prodromal symptoms of hepatitis, such as fatigue, weakness, malaise, anorexia, nausea or vomiting. If these symptoms appear or if signs suggestive of hepatic damage are detected, isoniazid should be discontinued promptly, since continued use of the drug in these cases has been reported to cause a more severe form of liver damage.
Care should be exercised in the treatment of elderly or malnourished patients who may also require vitamin B6 supplementation with the isoniazid therapy.
Use of isoniazid should be carefully monitored in patients with slow acetylator status, epilepsy, history of psychosis, history of peripheral neuropathy, diabetes, alcohol dependence, HIV infection or porphyria.
Effects on the Ability to Drive and Use Machines: Isoniazid has been associated with vertigo, visual disorders and psychotic reactions (see Adverse Reactions). Patients should be informed of these, and advised that if affected, they should not drive, operate machinery or take part in any activities where these symptoms may put either themselves or others at risk.
Use In Pregnancy & Lactation
Pregnancy: Rifampicin: Rifampicin has been shown to be teratogenic in rodents when given in large doses. There are no well controlled studies with Rifampicin + Isoniazid Fixed-Dose Combination in pregnant women. Although rifampicin has been reported to cross the placental barrier and appear in cord blood, the effect of rifampicin, alone or in combination with other antituberculosis drugs, on the human fetus is not known.
When administered during the last few weeks of pregnancy, rifampicin can cause post-natal hemorrhages in the mother and infant, for which treatment with Vitamin K1 may be indicated.
Isoniazid: It has been reported that in both rats and rabbits, isoniazid may exert an embryocardial effect when administered orally during pregnancy, although no isoniazid-related congenital anomalies have been found in reproduction studies in mammalian species (mice, rats, rabbits).
Therefore, Rifampicin + Isoniazid Fixed-Dose Combination should be used in pregnant women or in women of childbearing potential only if the potential benefit justifies the potential risk to the fetus.
Lactation: Rifampicin and isoniazid are excreted in breast milk and infants should not be breastfed by a patient receiving Rifampicin + Isoniazid Fixed-Dose Combination unless in the physician's judgement the potential benefit to the patient outweighs the potential risk to the infant.
In breast-fed infants whose mothers are taking isoniazid, there is a theoretical risk of convulsions and neuropathy (associated with vitamin B6 deficiency), therefore they should be monitored for early signs of these effects and consideration should be given to treating both mother and infant prophylactically with pyridoxine.
When administered during the last few weeks of pregnancy, rifampicin can cause post-natal hemorrhages in the mother and infant, for which treatment with Vitamin K1 may be indicated.
Isoniazid: It has been reported that in both rats and rabbits, isoniazid may exert an embryocardial effect when administered orally during pregnancy, although no isoniazid-related congenital anomalies have been found in reproduction studies in mammalian species (mice, rats, rabbits).
Therefore, Rifampicin + Isoniazid Fixed-Dose Combination should be used in pregnant women or in women of childbearing potential only if the potential benefit justifies the potential risk to the fetus.
Lactation: Rifampicin and isoniazid are excreted in breast milk and infants should not be breastfed by a patient receiving Rifampicin + Isoniazid Fixed-Dose Combination unless in the physician's judgement the potential benefit to the patient outweighs the potential risk to the infant.
In breast-fed infants whose mothers are taking isoniazid, there is a theoretical risk of convulsions and neuropathy (associated with vitamin B6 deficiency), therefore they should be monitored for early signs of these effects and consideration should be given to treating both mother and infant prophylactically with pyridoxine.
Adverse Reactions
Rifampicin: Reactions to rifampicin occurring with either daily or intermittent dosage regimens include: Cutaneous reactions which are mild and self-limiting may occur and do not appear to be hypersensitivity reactions. Typically, they consist of flushing and itching with or without a rash. Urticaria and more serious hypersensitivity reactions occur but are uncommon. Exfoliative dermatitis, pemphigoid reaction, erythema multiforme including Stevens-Johnson syndrome, toxic epidermal necrolysis, Lyell's syndrome and vasculitis have been reported rarely.
Gastrointestinal reactions consist of anorexia, nausea, vomiting, abdominal discomfort, and diarrhea. Pseudomembranous colitis has been reported with rifampicin therapy.
Hepatitis can be caused by rifampicin and liver function tests should be monitored (see Precautions).
Central Nervous System: Psychoses have been rarely reported.
Thrombocytopenia with or without purpura may occur, usually associated with intermittent therapy, but is reversible if drug is discontinued as soon as purpura occurs. Cerebral hemorrhage and fatalities have been reported when rifampicin administration has been continued or resumed after the appearance of purpura.
Disseminated intravascular coagulation has also been rarely reported.
Eosinophilia, leucopenia, oedema, muscle weakness and myopathy have been reported to occur in a small percentage of patients treated with rifampicin.
Agranulocytosis has been reported very rarely reported.
Rare reports of adrenal insufficiency in patients with compromised adrenal function have been observed.
Reactions usually occurring with intermittent dosage regimens and probably of immunological origin include: 'Flu Syndrome' consisting of episodes of fever, chills, headache, dizziness, and bone pain appearing most commonly during the 3rd to the 6th month of therapy. The frequency of the syndrome varies but may occur in up to 50 % of patients given once-weekly regimens with a dose of rifampicin of 25 mg/kg or more; Shortness of breath and wheezing; Decrease in blood pressure and shock; Anaphylaxis; Acute hemolytic anemia; Acute renal failure usually due to acute tubular necrosis or acute interstitial nephritis.
If serious complications arise e.g. renal failure, thrombocytopenia or hemolytic anemia, rifampicin should be stopped and never restarted.
Occasional disturbances of the menstrual cycle have been reported in women receiving long-term antituberculosis therapy with regimens containing rifampicin.
Rifampicin may produce a reddish coloration of the urine, sweat, sputum and tears. The patient should be forewarned of this. Soft contact lenses may be permanently stained.
Isoniazid: Hypersensitivity reactions: Fever, anaphylactic reactions.
Nervous system: Vertigo; polyneuritis, presenting as paresthesia, muscle weakness, loss of tendon reflexes, etc., is unlikely to occur with the recommended daily dose of Rifampicin + Isoniazid Fixed-Dose Combination. The incidence is higher in "slow acetylators". Other neurotoxic effects, which are uncommon with conventional doses, are convulsions, toxic encephalopathy, optic neuritis and atrophy, memory impairment and toxic psychosis. The possibility that the frequency of seizures may be increased in patients with epilepsy should be borne in mind.
Cutaneous: Rash, acne, Stevens-Johnson syndrome, exfoliative dermatitis and pemphigus.
Hematologic: Eosinophilia, agranulocytosis, thrombocytopenia, anemia, aplastic anemia and hemolytic anemia.
Gastrointestinal: Pancreatitis, constipation, dry mouth, nausea, vomiting and epigastric distress.
Hepatic: Severe and sometimes fatal hepatitis may occur with isoniazid therapy.
Reproductive System and Breast Disorders: Gynecomastia.
Investigations: Anti-nuclear antibodies.
Metabolism and Nutrition Disorders: Hyperglycemia.
Miscellaneous: Pellagra, systemic lupus erythematosus-like syndrome.
Gastrointestinal reactions consist of anorexia, nausea, vomiting, abdominal discomfort, and diarrhea. Pseudomembranous colitis has been reported with rifampicin therapy.
Hepatitis can be caused by rifampicin and liver function tests should be monitored (see Precautions).
Central Nervous System: Psychoses have been rarely reported.
Thrombocytopenia with or without purpura may occur, usually associated with intermittent therapy, but is reversible if drug is discontinued as soon as purpura occurs. Cerebral hemorrhage and fatalities have been reported when rifampicin administration has been continued or resumed after the appearance of purpura.
Disseminated intravascular coagulation has also been rarely reported.
Eosinophilia, leucopenia, oedema, muscle weakness and myopathy have been reported to occur in a small percentage of patients treated with rifampicin.
Agranulocytosis has been reported very rarely reported.
Rare reports of adrenal insufficiency in patients with compromised adrenal function have been observed.
Reactions usually occurring with intermittent dosage regimens and probably of immunological origin include: 'Flu Syndrome' consisting of episodes of fever, chills, headache, dizziness, and bone pain appearing most commonly during the 3rd to the 6th month of therapy. The frequency of the syndrome varies but may occur in up to 50 % of patients given once-weekly regimens with a dose of rifampicin of 25 mg/kg or more; Shortness of breath and wheezing; Decrease in blood pressure and shock; Anaphylaxis; Acute hemolytic anemia; Acute renal failure usually due to acute tubular necrosis or acute interstitial nephritis.
If serious complications arise e.g. renal failure, thrombocytopenia or hemolytic anemia, rifampicin should be stopped and never restarted.
Occasional disturbances of the menstrual cycle have been reported in women receiving long-term antituberculosis therapy with regimens containing rifampicin.
Rifampicin may produce a reddish coloration of the urine, sweat, sputum and tears. The patient should be forewarned of this. Soft contact lenses may be permanently stained.
Isoniazid: Hypersensitivity reactions: Fever, anaphylactic reactions.
Nervous system: Vertigo; polyneuritis, presenting as paresthesia, muscle weakness, loss of tendon reflexes, etc., is unlikely to occur with the recommended daily dose of Rifampicin + Isoniazid Fixed-Dose Combination. The incidence is higher in "slow acetylators". Other neurotoxic effects, which are uncommon with conventional doses, are convulsions, toxic encephalopathy, optic neuritis and atrophy, memory impairment and toxic psychosis. The possibility that the frequency of seizures may be increased in patients with epilepsy should be borne in mind.
Cutaneous: Rash, acne, Stevens-Johnson syndrome, exfoliative dermatitis and pemphigus.
Hematologic: Eosinophilia, agranulocytosis, thrombocytopenia, anemia, aplastic anemia and hemolytic anemia.
Gastrointestinal: Pancreatitis, constipation, dry mouth, nausea, vomiting and epigastric distress.
Hepatic: Severe and sometimes fatal hepatitis may occur with isoniazid therapy.
Reproductive System and Breast Disorders: Gynecomastia.
Investigations: Anti-nuclear antibodies.
Metabolism and Nutrition Disorders: Hyperglycemia.
Miscellaneous: Pellagra, systemic lupus erythematosus-like syndrome.
Drug Interactions
Food Interaction: Because isoniazid has some monoamine oxidase inhibiting activity, an interaction with tyramine-containing foods (cheese, red wine) may occur. Diamine oxidase may also be inhibited, causing exaggerated response (e.g. headache, sweating, palpitations, flushing, hypotension) to foods containing histamine (e.g. skipjack, tuna, other tropical fish). Tyramine- and histamine-containing foods should be avoided by patients receiving Rifampicin + Isoniazid.
Interactions with Other Medicinal Products: Cytochrome P-450 enzyme interaction: Rifampicin is known to induce and isoniazid is known to inhibit certain cytochrome P-450 enzymes. In general, the impact of the competing effects of rifampicin and isoniazid on the metabolism of drugs that undergo biotransformation through the affected pathways is unknown. Therefore, caution should be used when prescribing Rifampicin + Isoniazid Fixed-Dose Combination with drugs metabolized by cytochrome P-450. To maintain optimum therapeutic blood levels, dosages of drugs metabolized by these enzymes may require adjustment when starting or stopping Rifampicin + Isoniazid Fixed-Dose Combination.
Rifampicin: Rifampicin induces hepatic enzymes, and may increase the dosage requirements of drugs metabolized in the liver, including: Anti-infectives (including certain antiretroviral drugs, mefloquine, azole antifungal agents, clarithromycin, erythromycin, doxycycline, atovaquone, chloramphenicol); hormone therapy, including ethinylestradiol, norethindrone, tamoxifen, levothyroxine; methadone; warfarin; cyclosporine; corticosteroids; anticonvulsants (including phenytoin); cardiovascular agents including digoxin (in patients with renal insufficiency), digitoxin, verapamil, nifedipine, diltiazem, propranolol, metoprolol, enalapril, losartan, quinidine, mexiletine, tocainide, propafenone; theophylline; sulfonylurea hypoglycemics; hypolipidemics including simvastatin and fluvastatin; nortriptyline, haloperidol, quetiapine, benzodiazepines (including diazepam, triazolam), zolpidem, buspirone.
Since rifampicin reduces the effectiveness of oral contraceptives, women should be advised to choose between one of two options for contraception. Following consultation with a clinician, the patient may use an oral contraceptive pill containing a higher dose of estrogen (50 μg); alternatively, a nonhormonal method of contraception may be used throughout rifampicin treatment and for at least one month subsequently.
Current antiretroviral drugs (non-nucleoside reverse transcriptase inhibitors and protease inhibitors) interact with rifampicin. This may result in ineffectiveness of antiretroviral drugs, ineffective treatment of TB or an increased risk of drug toxicity.
Biliary excretion of radiocontrast media and sulfobromophthalein sodium may be reduced and microbiological assays for folic acid and vitamin B12 disturbed.
Other Interactions: When the two drugs were taken concomitantly, decreased concentrations of atovaquone and increased concentrations of rifampicin were observed.
Concurrent use of ketoconazole and rifampicin has resulted in decreased serum concentrations of both drugs.
Concurrent use of rifampicin and enalapril has resulted in decreased concentrations of enalaprilat, the active metabolite of enalapril. Dosage adjustments should be made if indicated by the patient's clinical condition.
Concomitant antacid administration may reduce the absorption of rifampicin. Daily doses of rifampicin should be given at least 1 hour before the ingestion of antacids.
When rifampicin is given concomitantly with either halothane or isoniazid, the potential for hepatotoxicity is increased. The concomitant use of rifampicin and halothane should be avoided. Patients receiving both rifampicin and isoniazid should be monitored closely for hepatotoxicity.
When rifampicin is taken with para-aminosalicylic acid (PAS), rifampicin levels in the serum may decrease. Therefore, the drugs should be taken at least eight hours apart.
Interactions with Isoniazid: Isoniazid inhibits the metabolism of certain drugs, which can increase their plasma concentration to the point of toxicity. Rifampicin, however, has the opposite effect for many of these drugs. For example, the available data indicate that administering both rifampicin and isoniazid causes a reduction in plasma levels of phenytoin and diazepam.
Isoniazid may increase the toxicity of carbamazepine, benzodiazepines metabolized by oxidation (such as triazolam), acetaminophen, valproate, serotonergic antidepressants, disulfiram, warfarin and theophylline.
Interference with laboratory and diagnostic tests: Therapeutic levels of rifampicin have been shown to inhibit standard microbiological assays for serum folate and Vitamin B12. Thus, alternative assay methods should be considered. Transient elevation of BSP and serum bilirubin has been reported. Rifampicin may impair biliary excretion of contrast media used for visualization of the gallbladder, due to competition for biliary excretion. Therefore, these tests should be performed before the morning dose of rifampicin.
Interactions with Other Medicinal Products: Cytochrome P-450 enzyme interaction: Rifampicin is known to induce and isoniazid is known to inhibit certain cytochrome P-450 enzymes. In general, the impact of the competing effects of rifampicin and isoniazid on the metabolism of drugs that undergo biotransformation through the affected pathways is unknown. Therefore, caution should be used when prescribing Rifampicin + Isoniazid Fixed-Dose Combination with drugs metabolized by cytochrome P-450. To maintain optimum therapeutic blood levels, dosages of drugs metabolized by these enzymes may require adjustment when starting or stopping Rifampicin + Isoniazid Fixed-Dose Combination.
Rifampicin: Rifampicin induces hepatic enzymes, and may increase the dosage requirements of drugs metabolized in the liver, including: Anti-infectives (including certain antiretroviral drugs, mefloquine, azole antifungal agents, clarithromycin, erythromycin, doxycycline, atovaquone, chloramphenicol); hormone therapy, including ethinylestradiol, norethindrone, tamoxifen, levothyroxine; methadone; warfarin; cyclosporine; corticosteroids; anticonvulsants (including phenytoin); cardiovascular agents including digoxin (in patients with renal insufficiency), digitoxin, verapamil, nifedipine, diltiazem, propranolol, metoprolol, enalapril, losartan, quinidine, mexiletine, tocainide, propafenone; theophylline; sulfonylurea hypoglycemics; hypolipidemics including simvastatin and fluvastatin; nortriptyline, haloperidol, quetiapine, benzodiazepines (including diazepam, triazolam), zolpidem, buspirone.
Since rifampicin reduces the effectiveness of oral contraceptives, women should be advised to choose between one of two options for contraception. Following consultation with a clinician, the patient may use an oral contraceptive pill containing a higher dose of estrogen (50 μg); alternatively, a nonhormonal method of contraception may be used throughout rifampicin treatment and for at least one month subsequently.
Current antiretroviral drugs (non-nucleoside reverse transcriptase inhibitors and protease inhibitors) interact with rifampicin. This may result in ineffectiveness of antiretroviral drugs, ineffective treatment of TB or an increased risk of drug toxicity.
Biliary excretion of radiocontrast media and sulfobromophthalein sodium may be reduced and microbiological assays for folic acid and vitamin B12 disturbed.
Other Interactions: When the two drugs were taken concomitantly, decreased concentrations of atovaquone and increased concentrations of rifampicin were observed.
Concurrent use of ketoconazole and rifampicin has resulted in decreased serum concentrations of both drugs.
Concurrent use of rifampicin and enalapril has resulted in decreased concentrations of enalaprilat, the active metabolite of enalapril. Dosage adjustments should be made if indicated by the patient's clinical condition.
Concomitant antacid administration may reduce the absorption of rifampicin. Daily doses of rifampicin should be given at least 1 hour before the ingestion of antacids.
When rifampicin is given concomitantly with either halothane or isoniazid, the potential for hepatotoxicity is increased. The concomitant use of rifampicin and halothane should be avoided. Patients receiving both rifampicin and isoniazid should be monitored closely for hepatotoxicity.
When rifampicin is taken with para-aminosalicylic acid (PAS), rifampicin levels in the serum may decrease. Therefore, the drugs should be taken at least eight hours apart.
Interactions with Isoniazid: Isoniazid inhibits the metabolism of certain drugs, which can increase their plasma concentration to the point of toxicity. Rifampicin, however, has the opposite effect for many of these drugs. For example, the available data indicate that administering both rifampicin and isoniazid causes a reduction in plasma levels of phenytoin and diazepam.
Isoniazid may increase the toxicity of carbamazepine, benzodiazepines metabolized by oxidation (such as triazolam), acetaminophen, valproate, serotonergic antidepressants, disulfiram, warfarin and theophylline.
Interference with laboratory and diagnostic tests: Therapeutic levels of rifampicin have been shown to inhibit standard microbiological assays for serum folate and Vitamin B12. Thus, alternative assay methods should be considered. Transient elevation of BSP and serum bilirubin has been reported. Rifampicin may impair biliary excretion of contrast media used for visualization of the gallbladder, due to competition for biliary excretion. Therefore, these tests should be performed before the morning dose of rifampicin.
Storage
Store at temperatures not exceeding 30°C.
Protect from heat, light and moisture.
Shelf-Life: 3 years.
Protect from heat, light and moisture.
Shelf-Life: 3 years.
Action
Pharmacological Category: Antituberculosis.
Pharmacology: Pharmacodynamics: Rifampicin and isoniazid are active bactericidal antituberculosis drugs which are particularly active against the rapidly growing extracellular organisms and also have bactericidal activity intracellularly. Rifampicin has activity against slow- and intermittently-growing M. tuberculosis.
Rifampicin inhibits DNA-dependent RNA polymerase activity in susceptible cells. Specifically, it interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme. Cross-resistance to rifampicin has only been shown with other rifamycins.
Isoniazid acts against actively growing tubercle bacilli.
Pharmacokinetics: Rifampicin: Rifampicin is readily absorbed from the stomach and the duodenum. Peak serum concentrations of the order of 10 μg/mL occur about 2-4 hours after a dose of 10 mg/kg body weight on an empty stomach.
In normal subjects the biological half-life of rifampicin in serum averages about 3 hours after a 600 mg dose and increases to 5.1 hours after a 900 mg dose. With repeated administration, the half-life decreases and reaches average value of approximately 2-3 hours. At a dose of up to 600 mg/day, the half-life does not differ in patients with renal failure and consequently, no dosage adjustment is required.
After absorption, rifampicin is rapidly eliminated in the bile and an enterohepatic circulation ensues. During this process, rifampicin undergoes progressive deacetylation, so that nearly all the drug in the bile is in this form in about 6 hours. This metabolite retains essentially complete antibacterial activity. Intestinal reabsorption is reduced by deacetylation and elimination is facilitated. Up to 30% of a dose is excreted in the urine, with about half of this being unchanged drug. Absorption of rifampicin is reduced when the drug is ingested with food.
Rifampicin is widely distributed throughout the body. It is present in effective concentrations in many organs and body fluids, including cerebrospinal fluid. Rifampicin is about 80% protein bound. Most of the unbound fraction is not ionized and therefore is diffused freely in tissues.
Isoniazid: After oral administration isoniazid produces peak blood levels within 1 to 2 hours which decline to 50% or less within 6 hours. Ingestion of isoniazid with food may reduce its absorption. It diffuses readily into all body fluids (cerebrospinal, pleural and ascitic fluids), tissues, organs and excreta (saliva, sputum and faeces). From 50 to 70% of a dose of isoniazid is excreted in the urine in 24 hours.
Isoniazid is metabolized primarily by acetylation and dehydrazination. The rate of acetylation is genetically determined.
Pharmacokinetic studies in normal volunteers have been shown that the two ingredients in Rifampicin + Isoniazid Fixed-Dose Combination comparable bioavailability whether they are given together as individual dose forms or as Rifampicin + Isoniazid Fixed-Dose Combination.
Toxicology: Preclinical Safety Data: Carcinogenesis: Isoniazid has been reported to induce pulmonary tumors in a number of strains of mice.
Pharmacology: Pharmacodynamics: Rifampicin and isoniazid are active bactericidal antituberculosis drugs which are particularly active against the rapidly growing extracellular organisms and also have bactericidal activity intracellularly. Rifampicin has activity against slow- and intermittently-growing M. tuberculosis.
Rifampicin inhibits DNA-dependent RNA polymerase activity in susceptible cells. Specifically, it interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme. Cross-resistance to rifampicin has only been shown with other rifamycins.
Isoniazid acts against actively growing tubercle bacilli.
Pharmacokinetics: Rifampicin: Rifampicin is readily absorbed from the stomach and the duodenum. Peak serum concentrations of the order of 10 μg/mL occur about 2-4 hours after a dose of 10 mg/kg body weight on an empty stomach.
In normal subjects the biological half-life of rifampicin in serum averages about 3 hours after a 600 mg dose and increases to 5.1 hours after a 900 mg dose. With repeated administration, the half-life decreases and reaches average value of approximately 2-3 hours. At a dose of up to 600 mg/day, the half-life does not differ in patients with renal failure and consequently, no dosage adjustment is required.
After absorption, rifampicin is rapidly eliminated in the bile and an enterohepatic circulation ensues. During this process, rifampicin undergoes progressive deacetylation, so that nearly all the drug in the bile is in this form in about 6 hours. This metabolite retains essentially complete antibacterial activity. Intestinal reabsorption is reduced by deacetylation and elimination is facilitated. Up to 30% of a dose is excreted in the urine, with about half of this being unchanged drug. Absorption of rifampicin is reduced when the drug is ingested with food.
Rifampicin is widely distributed throughout the body. It is present in effective concentrations in many organs and body fluids, including cerebrospinal fluid. Rifampicin is about 80% protein bound. Most of the unbound fraction is not ionized and therefore is diffused freely in tissues.
Isoniazid: After oral administration isoniazid produces peak blood levels within 1 to 2 hours which decline to 50% or less within 6 hours. Ingestion of isoniazid with food may reduce its absorption. It diffuses readily into all body fluids (cerebrospinal, pleural and ascitic fluids), tissues, organs and excreta (saliva, sputum and faeces). From 50 to 70% of a dose of isoniazid is excreted in the urine in 24 hours.
Isoniazid is metabolized primarily by acetylation and dehydrazination. The rate of acetylation is genetically determined.
Pharmacokinetic studies in normal volunteers have been shown that the two ingredients in Rifampicin + Isoniazid Fixed-Dose Combination comparable bioavailability whether they are given together as individual dose forms or as Rifampicin + Isoniazid Fixed-Dose Combination.
Toxicology: Preclinical Safety Data: Carcinogenesis: Isoniazid has been reported to induce pulmonary tumors in a number of strains of mice.
MedsGo Class
Anti-TB Agents
Features
Dosage
150 mg / 75 mg
Ingredients
- Isoniazid
- Rifampicin
Packaging
Film-Coated Tablet 1's
Generic Name
Isoniazid / Rifampicin
Registration Number
DRP-6598
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions


