Indications/Uses
Cefaclor is a cephalosporin antibiotic administered by mouth similarly to Cefalexin in the treatment of the following infections when caused by susceptible strains of the designated microorganisms: Lower respiratory tract infections, including pneumonia, acute bronchitis, acute exacerbations of chronic bronchitis caused by S. pneumoniae, H. influenzae, Staphylococci, S. pyogenes (group A β-hemolytic streptococci), and M. catarrhalis.
Upper respiratory infections, including pharyngitis and tonsillitis, caused by S. pyogenes (group A β-hemolytic streptococci), and M. catarrhalis.
Urinary tract infections, including pyelonephritis and cystitis, caused by E. coli, P. mirabilis, Klebsiella spp. and coagulase-negative Staphylococci.
Note: Cefaclor has been found to be effective in both acute and chronic urinary tract infections.
Skin and soft tissue infections caused by Staphylococcus aureus and S. pyogenes (group A β-hemolytic streptococci).
Otitis media caused by S. pneumoniae, H. influenzae, Staphylococci, S. pyogenes (group A β-hemolytic streptococci), and M. catarrhalis.
Cefaclor is classified as a second generation cephalosporin and its greater activity against Haemophilus influenzae makes it more suitable than cephalexin for the treatment of infections such as otitis media.
Sinusitis.
Gonococcal urethritis: Appropriate culture and susceptibility studies should be performed to determine susceptibility of the causative organism to Cefaclor.
Upper respiratory infections, including pharyngitis and tonsillitis, caused by S. pyogenes (group A β-hemolytic streptococci), and M. catarrhalis.
Urinary tract infections, including pyelonephritis and cystitis, caused by E. coli, P. mirabilis, Klebsiella spp. and coagulase-negative Staphylococci.
Note: Cefaclor has been found to be effective in both acute and chronic urinary tract infections.
Skin and soft tissue infections caused by Staphylococcus aureus and S. pyogenes (group A β-hemolytic streptococci).
Otitis media caused by S. pneumoniae, H. influenzae, Staphylococci, S. pyogenes (group A β-hemolytic streptococci), and M. catarrhalis.
Cefaclor is classified as a second generation cephalosporin and its greater activity against Haemophilus influenzae makes it more suitable than cephalexin for the treatment of infections such as otitis media.
Sinusitis.
Gonococcal urethritis: Appropriate culture and susceptibility studies should be performed to determine susceptibility of the causative organism to Cefaclor.
Dosage/Direction for Use
See table.
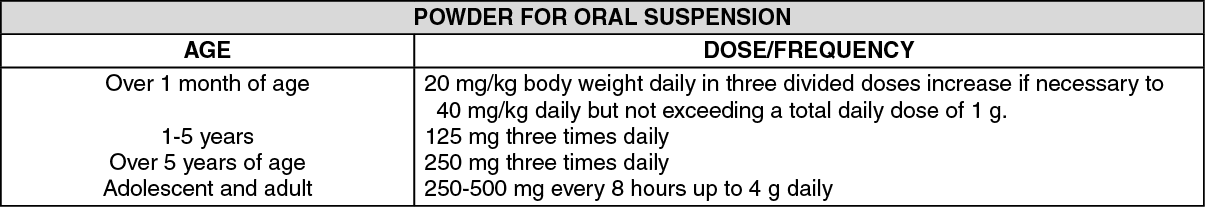
For bronchitis and pneumonia, the dosage is 250 mg administered three times daily. A dosage of 250 mg administered three times daily for 10 days is recommended for sinusitis. For more severe infections (such as pneumonia) or those caused by less susceptible organisms, doses may be doubled. Doses of 4 g per day have been administered safely to normal subjects for 28 days, but the total daily dosage should not exceed this amount. For the treatment of acute gonococcal urethritis in males and females, a single dose of 3 g combined with 1 g probenecid is given. Or as prescribed by the physician.
For children over 1 month, the usual recommended daily dosage is 20 mg/kg per day in divided doses every 8 hours. For bronchitis and pneumonia, the dosage is 20 mg/kg per day in divided doses administered three times daily. In more serious infections, otitis media and infections caused by less susceptible organisms, 40 mg/kg per day in divided doses are recommended, with a maximum dosage of 1 g per day or as prescribed by the physician.
For children over 1 month, the usual recommended daily dosage is 20 mg/kg per day in divided doses every 8 hours. For bronchitis and pneumonia, the dosage is 20 mg/kg per day in divided doses administered three times daily. In more serious infections, otitis media and infections caused by less susceptible organisms, 40 mg/kg per day in divided doses are recommended, with a maximum dosage of 1 g per day or as prescribed by the physician.
Administration
May be taken with or without food.
Contraindications
Cefaclor is contraindicated in patients with known allergy to the Cephalosporin group of antibiotics.
Warnings
For 250 mg/5 mL: This product contains FD & C Yellow #5 (Tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons.
Special Precautions
Prolonged use of Cefaclor may result in overgrowth of non-susceptible organisms and as with other broad spectrum antibiotics, pseudomembranous colitis may develop. Cefaclor should not be given to patients who are hypersensitive to it or to other cephalosporins. About 10% of penicillin-sensitive patients may also be allergic to cephalosporins. Great care should be taken if Cefaclor is to be given to such patients. Care is also necessary in patients with known histories of allergy. Cefaclor should be given with caution to patients with renal impairment a dosage reduction may be necessary. Renal and haematological status should be monitored especially during prolonged and high-dose therapy.
Adverse Reactions
The most common are hypersensitivity reactions including skin rashes, urticaria, eosinophilia fever, reactions resembling serum sickness and anaphylaxis.
There may be a positive response to the Coombs test although haemolytic anemia rarely occurs. Neutropenia and thrombocytopenia have occasionally been reported. Agranulocytosis has been associated rarely with some cephalosporins. Acute renal tubular necrosis has followed excessive dosage and has also been associated with its use in older patients or those with pre-existing renal impairment, or with the concomitant administration of nephrotoxic drugs such as aminoglycoside antibiotics. Acute interstitial nephritis is also a possibility as a manifestation of hypersensitivity. Transient increases in liver enzyme values have been reported. Hepatitis and cholestatic jaundice have occurred rarely with some cephalosporins. Convulsions and other signs of CNS toxicity have been associated with high doses, especially in patients with renal failure. Gastrointestinal adverse effects such as nausea, vomiting and diarrhea have been reported rarely. Prolonged use may result in overgrowth of non-susceptible organisms and, as with other broad-spectrum antibiotics, pseudomembranous colitis may develop.
There may be a positive response to the Coombs test although haemolytic anemia rarely occurs. Neutropenia and thrombocytopenia have occasionally been reported. Agranulocytosis has been associated rarely with some cephalosporins. Acute renal tubular necrosis has followed excessive dosage and has also been associated with its use in older patients or those with pre-existing renal impairment, or with the concomitant administration of nephrotoxic drugs such as aminoglycoside antibiotics. Acute interstitial nephritis is also a possibility as a manifestation of hypersensitivity. Transient increases in liver enzyme values have been reported. Hepatitis and cholestatic jaundice have occurred rarely with some cephalosporins. Convulsions and other signs of CNS toxicity have been associated with high doses, especially in patients with renal failure. Gastrointestinal adverse effects such as nausea, vomiting and diarrhea have been reported rarely. Prolonged use may result in overgrowth of non-susceptible organisms and, as with other broad-spectrum antibiotics, pseudomembranous colitis may develop.
Drug Interactions
Positive results to the direct Coombs test have been found during treatment with cephalosporins and these can interfere with blood cross matching. The urine of patients being treated with Cefaclor may give false-positive reactions for glucose using copper-reduction reactions.
Caution For Usage
Directions for Reconstitution: To make a 60 mL suspension, add 38 mL of water to 250 mg/5 mL Cefaclor Powder for Oral Suspension.
To make 20 mL suspension, add 14 mL of water to 50 mg/mL Cefaclor Powder for Oral Suspension.
Shake well until the contents are evenly suspended. The reconstituted suspension is stable for 14 days when refrigerated (2-8°C) and 7 days if stored at temperatures not exceeding 30°C.
To make 20 mL suspension, add 14 mL of water to 50 mg/mL Cefaclor Powder for Oral Suspension.
Shake well until the contents are evenly suspended. The reconstituted suspension is stable for 14 days when refrigerated (2-8°C) and 7 days if stored at temperatures not exceeding 30°C.
Storage
Store at temperatures not exceeding 30°C.
Action
Pharmacology: Pharmacokinetics: Cefaclor is well absorbed from the gastrointestinal tract but plasma concentrations are slightly lower than those achieved with Cephalexin or Cephradine. Doses of 250, 500 and 1000 mg by mouth produce peak plasma concentrations of about 6, 13, 23 µg per mL respectively at 0.5 to 1 hour. The presence of food may delay the absorption of Cefaclor, but the total amount absorbed is unchanged. A plasma half-life of 0.5 to 1 hour has been reported; it may be slightly prolonged in renal failure. About 25% is bound to plasma proteins. It is rapidly excreted by the kidneys; up to 85% of a dose appears unchanged in the urine within 8 hours, the greater part within 2 hours. High concentration of 600, 900 and 1,900 µg per mL have been reported after doses of 250, 500 and 1,000 mg respectively. Probenecid delays excretion. Cefaclor is removed by haemodialysis.
Microbiology: Cefaclor is bactericidal and has antimicrobial activity but is reported to be more active against Gram-negative bacteria including Escherichia coli, Klebsiella pneumoniae, Neisseria gonorrhea, and Proteus mirabilis especially against Haemophilus influenzae. It is active against some beta-lactamase-producing strains of H. influenzae. It may be less resistant to staphylococcal penicillinase than cephalexin or cephradine and a marked inoculum effect has been reported in vitro.
Microbiology: Cefaclor is bactericidal and has antimicrobial activity but is reported to be more active against Gram-negative bacteria including Escherichia coli, Klebsiella pneumoniae, Neisseria gonorrhea, and Proteus mirabilis especially against Haemophilus influenzae. It is active against some beta-lactamase-producing strains of H. influenzae. It may be less resistant to staphylococcal penicillinase than cephalexin or cephradine and a marked inoculum effect has been reported in vitro.
MedsGo Class
Cephalosporins
Features
Dosage
50mg /ml
Ingredients
- Cefaclor
Packaging
Suspension (Pediatric Oral Drops) 20ml
Generic Name
Cefaclor
Registration Number
DRP-2101
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions


