Indications/Uses
Tablet: For the treatment of the following infections caused by susceptible microorganisms: Upper respiratory tract infections (including ENT infections): Tonsillitis, sinusitis, otitis media.
Lower respiratory tract infections: Acute and chronic bronchitis, pneumonia, lung abscess.
Genito-urinary tract and abdominal infections: Cystitis, urethritis, pyelonephritis, female genital infections, septic abortion, pelvic or puerperal sepsis, intra-abdominal sepsis.
Skin and skin structure infections: Furuncle and abscess, cellulitis, wound infections.
Bone and joint infections: Osteomyelitis.
Dental infection: Dentoalveolar abscess.
Other infections: Septicemia, peritonitis, post-surgical infections.
Step down treatment for infections due to susceptible organisms, initially given antimicrobial therapy, particularly parenteral Co-amoxiclav.
Powder for injection: For the short-term treatment of the following infections caused by susceptible microorganisms: Upper respiratory tract infections (including ENT infections): Tonsillitis, sinusitis, otitis media.
Lower respiratory tract infections: acute exacerbations of chronic bronchitis, lobar and bronchopneumonia.
Genito-urinary tract and abdominal infections: cystitis, urethritis, pyelonephritis, female genital infections, septic abortion, puerperal sepsis, intra-abdominal sepsis.
Skin and skin structure infections: cellulitis, animal bites, severe dental abscess with spreading cellulitis.
Bone and joint infections: Osteomyelitis.
Other infections: Septicemia, peritonitis, post-surgical infections.
As prophylaxis against infections which may be associated with major surgical procedures such as gastrointestinal, pelvic, head and neck, cardiac, renal, joint replacement and biliary tract surgery.
Lower respiratory tract infections: Acute and chronic bronchitis, pneumonia, lung abscess.
Genito-urinary tract and abdominal infections: Cystitis, urethritis, pyelonephritis, female genital infections, septic abortion, pelvic or puerperal sepsis, intra-abdominal sepsis.
Skin and skin structure infections: Furuncle and abscess, cellulitis, wound infections.
Bone and joint infections: Osteomyelitis.
Dental infection: Dentoalveolar abscess.
Other infections: Septicemia, peritonitis, post-surgical infections.
Step down treatment for infections due to susceptible organisms, initially given antimicrobial therapy, particularly parenteral Co-amoxiclav.
Powder for injection: For the short-term treatment of the following infections caused by susceptible microorganisms: Upper respiratory tract infections (including ENT infections): Tonsillitis, sinusitis, otitis media.
Lower respiratory tract infections: acute exacerbations of chronic bronchitis, lobar and bronchopneumonia.
Genito-urinary tract and abdominal infections: cystitis, urethritis, pyelonephritis, female genital infections, septic abortion, puerperal sepsis, intra-abdominal sepsis.
Skin and skin structure infections: cellulitis, animal bites, severe dental abscess with spreading cellulitis.
Bone and joint infections: Osteomyelitis.
Other infections: Septicemia, peritonitis, post-surgical infections.
As prophylaxis against infections which may be associated with major surgical procedures such as gastrointestinal, pelvic, head and neck, cardiac, renal, joint replacement and biliary tract surgery.
Dosage/Direction for Use
Tablet: Administer Co-amoxiclav at the start of a meal to minimize potential gastrointestinal intolerance and to optimize absorption.
Drink plenty of water to ensure proper state of hydration and adequate urinary output.
Treatment should not exceed 14 days without first re-evaluating the patient.
Usual Oral Dosage in Adults and Children over 12 years old: See Table 4.
Drink plenty of water to ensure proper state of hydration and adequate urinary output.
Treatment should not exceed 14 days without first re-evaluating the patient.
Usual Oral Dosage in Adults and Children over 12 years old: See Table 4.
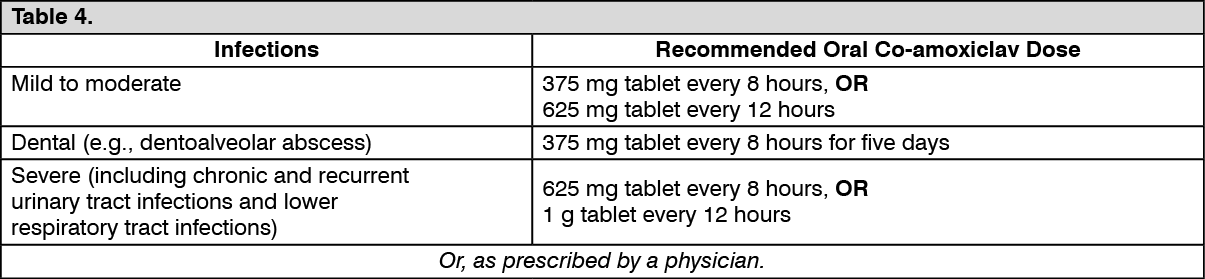
Dosage in Adults and Children over 12 years old with Renal Impairment: See Table 5.
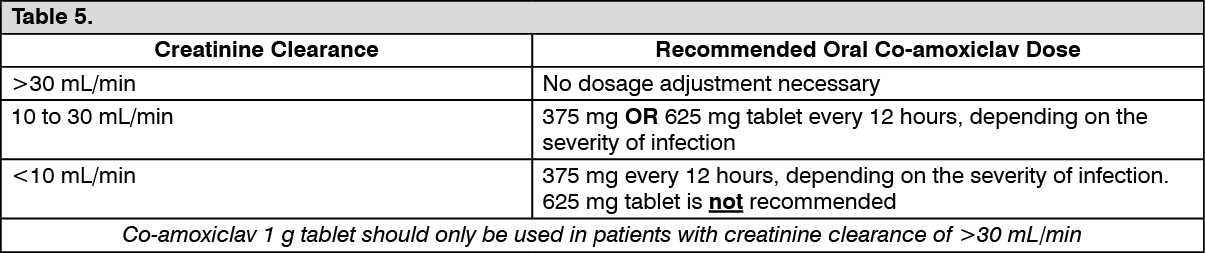
Dosage in Adults and Children over 12 years old on Hemodialysis: Co-amoxiclav 375 mg OR 625 mg tablet every 24 hours, depending on the severity of infection. Patients should receive an additional dose both during and at the end of dialysis.
Dosage in Adults and Children over 12 years old with Hepatic Impairment: Dose with caution and monitor hepatic function regularly.
Powder for injection: Co-amoxiclav may be administered either by IV injection or by intermittent infusion. It is NOT suitable for intramuscular (IM) administration.
Co-amoxiclav should be given by slow IV injection over a period of 3 to 4 minutes and within 20 minutes of reconstitution. It may be injected into the vein or via drip tube.
Treatment should not exceed 14 days without re-evaluation of the patient.
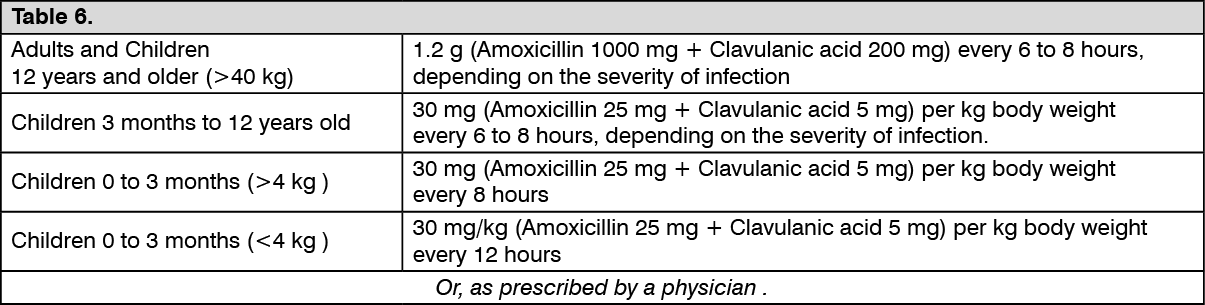
Usual Recommended IV Co-amoxiclav Dose: (See Table 6.)
Dosage in Adults and Children over 12 years old with Hepatic Impairment: Dose with caution and monitor hepatic function regularly.
Powder for injection: Co-amoxiclav may be administered either by IV injection or by intermittent infusion. It is NOT suitable for intramuscular (IM) administration.
Co-amoxiclav should be given by slow IV injection over a period of 3 to 4 minutes and within 20 minutes of reconstitution. It may be injected into the vein or via drip tube.
Treatment should not exceed 14 days without re-evaluation of the patient.
Usual Recommended IV Co-amoxiclav Dose: (See Table 6.)
Dosage for Surgical Prophylaxis: Surgical prophylaxis with Co-amoxiclav should aim to protect the patient for the period of risk of infection.
In adults, procedures lasting less than 1 hour are successfully covered by IV Co-amoxiclav 1.2 g (Amoxicillin 1000 mg + Clavulanic acid 200 mg) administered at induction of anesthesia.
Longer operations or when there is a high risk of infection (e.g., colorectal surgery) may require subsequent doses of IV Co-amoxiclav 1.2 g (Amoxicillin 1000 mg + Clavulanic acid 200 mg) [up to 4 doses in 24 hours], and this regimen can be continued for several days if the procedure has significantly increased the risk of infection.
Clear clinical signs of infection at operation will require a normal course of IV or oral Co-amoxiclav therapy post-operatively.
Dosage in Elderly Patients: No dosage adjustment necessary. However, if there is evidence of renal impairment, dose should be adjusted as for renally-impaired adults.
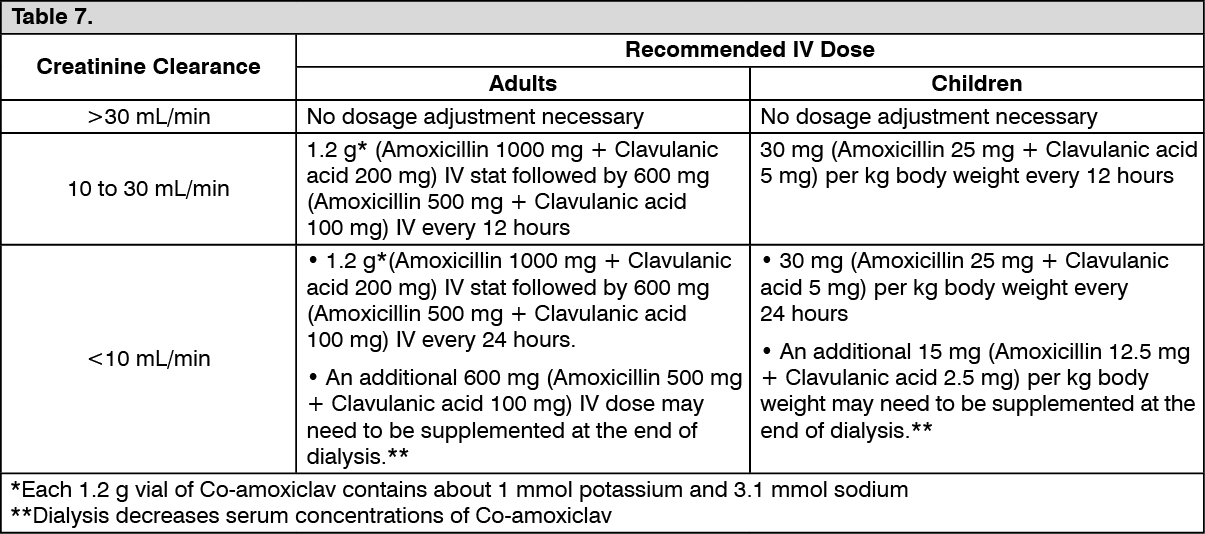
Dosage in Patients with Renal Impairment: Dosing adjustments are based on the maximum recommended level of amoxicillin. (See Table 7.)
In adults, procedures lasting less than 1 hour are successfully covered by IV Co-amoxiclav 1.2 g (Amoxicillin 1000 mg + Clavulanic acid 200 mg) administered at induction of anesthesia.
Longer operations or when there is a high risk of infection (e.g., colorectal surgery) may require subsequent doses of IV Co-amoxiclav 1.2 g (Amoxicillin 1000 mg + Clavulanic acid 200 mg) [up to 4 doses in 24 hours], and this regimen can be continued for several days if the procedure has significantly increased the risk of infection.
Clear clinical signs of infection at operation will require a normal course of IV or oral Co-amoxiclav therapy post-operatively.
Dosage in Elderly Patients: No dosage adjustment necessary. However, if there is evidence of renal impairment, dose should be adjusted as for renally-impaired adults.
Dosage in Patients with Renal Impairment: Dosing adjustments are based on the maximum recommended level of amoxicillin. (See Table 7.)
Dosage in Patients with Hepatic Impairment: Dose with caution; monitor hepatic function at regular intervals for both adults and children.
Data on where to base the dosage recommendation have not been established.
Data on where to base the dosage recommendation have not been established.
Overdosage
Clinical features of overdosage with Co-amoxiclav may include gastrointestinal symptoms, fluid and electrolyte imbalance. Amoxicillin crystalluria, leading to renal failure, has also been observed in some cases. Convulsions may occur in patients with impaired renal function or those receiving high doses.
Children (Additional Statement): A prospective study of 51 pediatric patients at a poison control center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms and do not require gastric emptying.
Symptomatic treatment is recommended. Co-amoxiclav can be removed by hemodialysis.
Children (Additional Statement): A prospective study of 51 pediatric patients at a poison control center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms and do not require gastric emptying.
Symptomatic treatment is recommended. Co-amoxiclav can be removed by hemodialysis.
Administration
May be taken with or without food: Best taken at the start of meals for better absorption & to reduce GI discomfort.
Contraindications
Hypersensitivity to penicillin or any component of the product; History of severe immediate hypersensitivity reaction (e.g., anaphylaxis or Stevens-Johnson syndrome) to another β-lactam antibiotic (e.g. a cephalosporin, carbapenem or monobactam); Patients with a previous history of cholestatic jaundice/hepatic impairment associated with Co-amoxiclav or penicillin; Patients with glandular fever or lymphatic lymphoma should not be given Co-amoxiclav as the amoxicillin component is likely to cause a maculopapular rash.
Special Precautions
Hypersensitivity Reactions: Serious and occasionally fatal hypersensitivity reactions (including anaphylactoid and severe cutaneous adverse reactions) have been reported in patients on penicillin therapy. Careful inquiry should be made concerning previous hypersensitivity to penicillins, cephalosporins, or other drugs before initiating therapy with Co-amoxiclav. Serious anaphylactic reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management, including intubation, should also be instituted.
Clostridium difficile-associated disease (CDAD): This has been reported with the use of nearly all antibacterial agents, including Co-amoxiclav, and may range in severity from mild diarrhea to fatal colitis. It is important to consider this diagnosis in patients who present with diarrhea, or symptoms of colitis, pseudomembranous colitis, toxic megacolon, or perforation of colon subsequent to the administration of any antibacterial agents. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
Hepatic Dysfunction: Hepatic dysfunction, including hepatitis and cholestatic jaundice has been associated with the use of Co-amoxiclav. Hepatic toxicity is usually reversible; however, deaths have been reported. Hepatic function should be monitored at regular intervals in patients with hepatic impairment.
In patients with renal impairment, the dose of Co-amoxiclav should be adjusted based on the degree of impairment [see Dosage & Administration].
During administration of high doses of amoxicillin, it is recommended to maintain adequate fluid intake and urinary output in order to reduce the possibility of crystalluria associated with amoxicillin therapy.
Appropriate monitoring should be undertaken when anticoagulants are prescribed concomitantly with Co-amoxiclav since prolongation of prothrombin time has been reported rarely in patients receiving Co-amoxiclav.
Although Co-amoxiclav has a low toxicity profile, the renal, hepatic, and hematopoietic status of patients undergoing prolonged treatment with the drug should be evaluated periodically.
Co-amoxiclav should be avoided if infectious mononucleosis is suspected since the occurrence of a morbilliform rash has been associated with this condition following the use of amoxicillin.
As with other antibacterial drugs, long term or repeated use may result in overgrowth of non-susceptible organisms, including fungi.
Carcinogenesis, Mutagenesis, Impairment of Fertility: The carcinogenic potential of Co-amoxiclav has not been evaluated in long-term animal studies.
The mutagenic potential of the drug has been investigated in vitro using Ames test, a human lymphocyte cytogenic assay, a yeast test and a mouse lymphoma forward mutation assay, and in vivo using mouse micronucleus tests and a dominant lethal test. Results were negative for all tests except for the in vitro mouse lymphoma assay where weak activity was found at very high, cytotoxic concentrations.
Oral doses of Co-amoxiclav up to 1,200 mg/kg/day (5.7 times the maximum human dose) was found to have no effect on fertility and reproductive performance in rats, dosed with a 2:1 ratio formulation of amoxicillin:clavulanic acid.
Effects on Ability to Drive and Use Machines: No studies on the effects on the ability to drive and use machines have been performed. However, undesirable effects may occur (e.g., allergic reactions, dizziness and convulsions), which may influence the ability to drive and use machines.
Renal Impairment: Dosage adjustments are based on the maximum recommended level of amoxicillin. No adjustment in dose is required in patients with creatinine clearance greater than 30 mL/min.
Hepatic Impairment: Dose with caution; monitor hepatic function at regular intervals for both adults and children.
Use in Children: Children weighing over 40 kg should be dosed based on recommended dosing in adult patients. The safety and efficacy of Co-amoxiclav tablets in children weighing less than 40 kg have not been established.
Use in the Elderly: Since elderly patients have increased risk of renal impairment, dose adjustment and renal function monitoring may be necessary.
Clostridium difficile-associated disease (CDAD): This has been reported with the use of nearly all antibacterial agents, including Co-amoxiclav, and may range in severity from mild diarrhea to fatal colitis. It is important to consider this diagnosis in patients who present with diarrhea, or symptoms of colitis, pseudomembranous colitis, toxic megacolon, or perforation of colon subsequent to the administration of any antibacterial agents. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
Hepatic Dysfunction: Hepatic dysfunction, including hepatitis and cholestatic jaundice has been associated with the use of Co-amoxiclav. Hepatic toxicity is usually reversible; however, deaths have been reported. Hepatic function should be monitored at regular intervals in patients with hepatic impairment.
In patients with renal impairment, the dose of Co-amoxiclav should be adjusted based on the degree of impairment [see Dosage & Administration].
During administration of high doses of amoxicillin, it is recommended to maintain adequate fluid intake and urinary output in order to reduce the possibility of crystalluria associated with amoxicillin therapy.
Appropriate monitoring should be undertaken when anticoagulants are prescribed concomitantly with Co-amoxiclav since prolongation of prothrombin time has been reported rarely in patients receiving Co-amoxiclav.
Although Co-amoxiclav has a low toxicity profile, the renal, hepatic, and hematopoietic status of patients undergoing prolonged treatment with the drug should be evaluated periodically.
Co-amoxiclav should be avoided if infectious mononucleosis is suspected since the occurrence of a morbilliform rash has been associated with this condition following the use of amoxicillin.
As with other antibacterial drugs, long term or repeated use may result in overgrowth of non-susceptible organisms, including fungi.
Carcinogenesis, Mutagenesis, Impairment of Fertility: The carcinogenic potential of Co-amoxiclav has not been evaluated in long-term animal studies.
The mutagenic potential of the drug has been investigated in vitro using Ames test, a human lymphocyte cytogenic assay, a yeast test and a mouse lymphoma forward mutation assay, and in vivo using mouse micronucleus tests and a dominant lethal test. Results were negative for all tests except for the in vitro mouse lymphoma assay where weak activity was found at very high, cytotoxic concentrations.
Oral doses of Co-amoxiclav up to 1,200 mg/kg/day (5.7 times the maximum human dose) was found to have no effect on fertility and reproductive performance in rats, dosed with a 2:1 ratio formulation of amoxicillin:clavulanic acid.
Effects on Ability to Drive and Use Machines: No studies on the effects on the ability to drive and use machines have been performed. However, undesirable effects may occur (e.g., allergic reactions, dizziness and convulsions), which may influence the ability to drive and use machines.
Renal Impairment: Dosage adjustments are based on the maximum recommended level of amoxicillin. No adjustment in dose is required in patients with creatinine clearance greater than 30 mL/min.
Hepatic Impairment: Dose with caution; monitor hepatic function at regular intervals for both adults and children.
Use in Children: Children weighing over 40 kg should be dosed based on recommended dosing in adult patients. The safety and efficacy of Co-amoxiclav tablets in children weighing less than 40 kg have not been established.
Use in the Elderly: Since elderly patients have increased risk of renal impairment, dose adjustment and renal function monitoring may be necessary.
Use In Pregnancy & Lactation
Pregnancy: Pregnancy Category B. There are no adequate or well-controlled studies in pregnant women. The safe use in pregnancy has not been established. However, oral Co-amoxiclav has been administered to pregnant women, particularly in the treatment of urinary tract infections, without evidence of adverse effects to the fetus.
In a single study in women with preterm, premature rupture of the fetal membrane (pPROM), it was reported that prophylactic treatment with Co-amoxiclav may be associated with an increased risk of necrotizing enterocolitis in neonates. Co-amoxiclav use should be avoided in pregnancy unless considered essential by the physician.
Lactation: Co-amoxiclav is distributed in human milk and may cause diarrhea, fungal infection of the mucous membrane and sensitization in the breastfed infant. Caution should be exercised if Co-amoxiclav is to be administered to a nursing mother.
In a single study in women with preterm, premature rupture of the fetal membrane (pPROM), it was reported that prophylactic treatment with Co-amoxiclav may be associated with an increased risk of necrotizing enterocolitis in neonates. Co-amoxiclav use should be avoided in pregnancy unless considered essential by the physician.
Lactation: Co-amoxiclav is distributed in human milk and may cause diarrhea, fungal infection of the mucous membrane and sensitization in the breastfed infant. Caution should be exercised if Co-amoxiclav is to be administered to a nursing mother.
Adverse Reactions
The most frequently reported adverse effects with co-amoxiclav include diarrhea, nausea and vomiting.
Infections and infestations: Mucocutaneous candidiasis, overgrowth of non-susceptible organisms.
Blood and lymphatic system disorders: Agranulocytosis, anemia, bleeding time prolonged, hemolytic anemia, eosinophilia, leukopenia, neutropenia, prothrombin time prolonged, thrombocytopenia, thrombocytopenia purpura.
Immune system disorders: Anaphylaxis, angioneurotic edema, serum sickness-like syndrome.
Metabolism and nutrition disorders: Anorexia.
Psychiatric disorders: Abnormal behavior, anxiety, reversible hyperactivity.
Nervous system disorders: Agitation, aseptic meningitis, confusion, convulsions, dizziness, headache, insomnia, reversible hyperactivity.
Vascular disorders: hypersensitivity vasculitis.
Gastrointestinal disorders: Abdominal discomfort, black hairy tongue, Clostridium difficile-associated diarrhea and colitis, hemorrhagic colitis, dyspepsia, enterocolitis, flatulence, gastritis, glossitis, indigestion, pseudomembranous colitis, stomatitis, tooth discoloration (brown, yellow or gray staining which is very rare in children).
Hepatobiliary disorders: Cholestatic jaundice, hepatic dysfunction, hepatitis, increases in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and serum bilirubin.
Skin and subcutaneous tissue disorders: Acute generalized exanthematous pustulosis (AGEP), bullous exfoliative-dermatitis, drug reaction with eosinophilia and systemic symptoms (DRESS), erythema multiforme, hypersensitivity vasculitis, maculopapular rash, pruritus, skin rash, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria.
Renal and urinary disorders: Crystalluria, interstitial nephritis.
Reproductive system and breast disorders: Vaginitis.
General disorders and administration site conditions: Fever, hematuria.
Infections and infestations: Mucocutaneous candidiasis, overgrowth of non-susceptible organisms.
Blood and lymphatic system disorders: Agranulocytosis, anemia, bleeding time prolonged, hemolytic anemia, eosinophilia, leukopenia, neutropenia, prothrombin time prolonged, thrombocytopenia, thrombocytopenia purpura.
Immune system disorders: Anaphylaxis, angioneurotic edema, serum sickness-like syndrome.
Metabolism and nutrition disorders: Anorexia.
Psychiatric disorders: Abnormal behavior, anxiety, reversible hyperactivity.
Nervous system disorders: Agitation, aseptic meningitis, confusion, convulsions, dizziness, headache, insomnia, reversible hyperactivity.
Vascular disorders: hypersensitivity vasculitis.
Gastrointestinal disorders: Abdominal discomfort, black hairy tongue, Clostridium difficile-associated diarrhea and colitis, hemorrhagic colitis, dyspepsia, enterocolitis, flatulence, gastritis, glossitis, indigestion, pseudomembranous colitis, stomatitis, tooth discoloration (brown, yellow or gray staining which is very rare in children).
Hepatobiliary disorders: Cholestatic jaundice, hepatic dysfunction, hepatitis, increases in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and serum bilirubin.
Skin and subcutaneous tissue disorders: Acute generalized exanthematous pustulosis (AGEP), bullous exfoliative-dermatitis, drug reaction with eosinophilia and systemic symptoms (DRESS), erythema multiforme, hypersensitivity vasculitis, maculopapular rash, pruritus, skin rash, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria.
Renal and urinary disorders: Crystalluria, interstitial nephritis.
Reproductive system and breast disorders: Vaginitis.
General disorders and administration site conditions: Fever, hematuria.
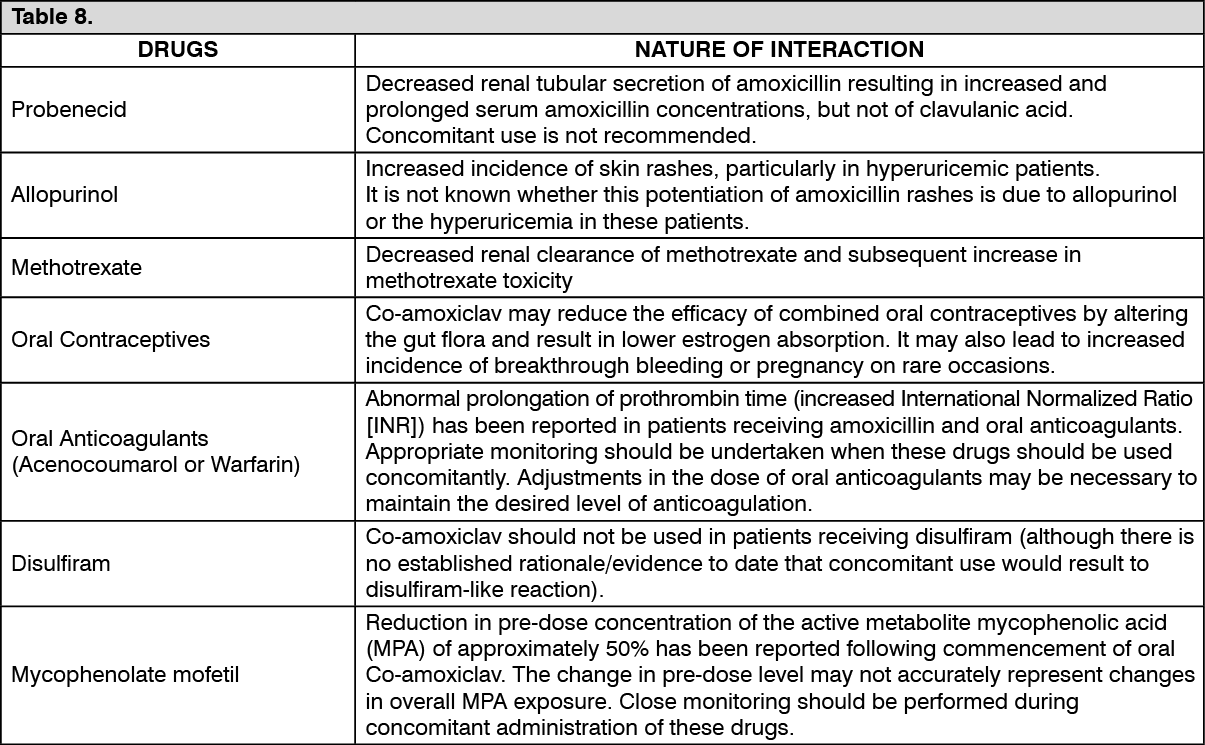
Drug Interactions
See Table 8.
Interference with Laboratory Tests: High urine concentrations of ampicillin may result in false-positive reactions when testing for urinary glucose using cupric sulfate (e.g., Clinitest, Benedict's Solution). Since this effect may also occur with amoxicillin, glucose oxidase methods (e.g., Clinistix) should be used when urinary glucose determinations are indicated in patients receiving Co-amoxiclav.
Although not reported to date with Co-amoxiclav, positive direct antiglobulin (Coombs') test results have been reported in patients who received ticarcillin and clavulanic acid and appear to be caused by clavulanic acid. This reaction may interfere with hematologic studies or transfusion cross-matching procedures and therefore should be considered in patients receiving Co-amoxiclav.
Following administration of amoxicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estriol, estriol-glucuronide, conjugated estrone, and estradiol has been noted.
Although not reported to date with Co-amoxiclav, positive direct antiglobulin (Coombs') test results have been reported in patients who received ticarcillin and clavulanic acid and appear to be caused by clavulanic acid. This reaction may interfere with hematologic studies or transfusion cross-matching procedures and therefore should be considered in patients receiving Co-amoxiclav.
Following administration of amoxicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estriol, estriol-glucuronide, conjugated estrone, and estradiol has been noted.
Caution For Usage
Powder for injection: Directions for Use: Directions for Reconstitution: To reconstitute, dissolve IV Co-amoxiclav powder in the required amount of diluent (Water for injection is the usual diluent). (See Table 9.)
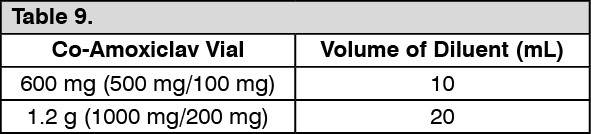
There are two ways of administering IV Co-amoxiclav: Slow IV injection for 3 to 4 minutes after reconstitution.
IV infusion over 30 to 40 minutes (should be further diluted after reconstitution).
Preparation of IV Infusion and Stability: Add without delay (i.e., Solutions should be made up to full infusion volume immediately after reconstitution) Co-amoxiclav 600 mg (500 mg/100 mg) reconstituted solution to 50 mL infusion fluid OR 1.2 g (1000 mg/200 mg) reconstituted solution to 100 mL infusion fluid (preferably using a mini-bag or in-line burette). Infuse over 30 to 40 minutes and complete within the time stated.
Satisfactory antibiotic concentrations are retained at 5°C and at room temperature (25°C) in the recommended volumes of the following infusion fluids. If reconstituted and maintained at room temperature, infusions should be completed within the time stated. (See Table 10.)
IV infusion over 30 to 40 minutes (should be further diluted after reconstitution).
Preparation of IV Infusion and Stability: Add without delay (i.e., Solutions should be made up to full infusion volume immediately after reconstitution) Co-amoxiclav 600 mg (500 mg/100 mg) reconstituted solution to 50 mL infusion fluid OR 1.2 g (1000 mg/200 mg) reconstituted solution to 100 mL infusion fluid (preferably using a mini-bag or in-line burette). Infuse over 30 to 40 minutes and complete within the time stated.
Satisfactory antibiotic concentrations are retained at 5°C and at room temperature (25°C) in the recommended volumes of the following infusion fluids. If reconstituted and maintained at room temperature, infusions should be completed within the time stated. (See Table 10.)
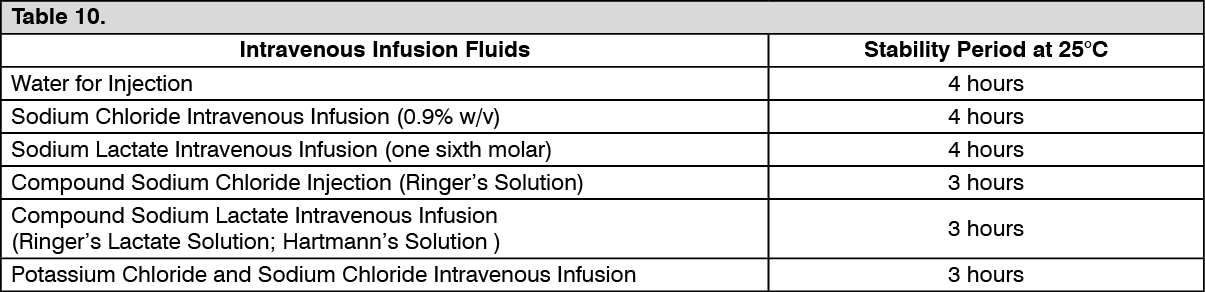
Reconstituted solution should not be frozen.
For storage at 5°C, the reconstituted solution should be added to pre-refrigerated infusion bags which may be stored for up to 8 hours.
Thereafter, the infusion should be administered immediately after reaching room temperature. (See Table 11.)
For storage at 5°C, the reconstituted solution should be added to pre-refrigerated infusion bags which may be stored for up to 8 hours.
Thereafter, the infusion should be administered immediately after reaching room temperature. (See Table 11.)

Co-amoxiclav vials are not suitable for multi-dose use. Any unused antibiotic should be disposed properly.
Co-amoxiclav IV is less stable in infusions containing glucose, dextran or bicarbonate. Therefore, reconstituted solution should not be added to such infusions but may be injected into the drip tubing over a period of 3 to 4 minutes.
Incompatibilities: Co-amoxiclav IV should not be mixed with blood products, other proteinaceous fluids such as protein hydrolysates or with IV lipid emulsions. If Co-amoxiclav injection is prescribed concomitantly with an aminoglycoside, the antibiotics should not be mixed in the syringe, IV fluid or giving set because of loss of activity of the aminoglycoside under these conditions.
Co-amoxiclav IV is less stable in infusions containing glucose, dextran or bicarbonate. Therefore, reconstituted solution should not be added to such infusions but may be injected into the drip tubing over a period of 3 to 4 minutes.
Incompatibilities: Co-amoxiclav IV should not be mixed with blood products, other proteinaceous fluids such as protein hydrolysates or with IV lipid emulsions. If Co-amoxiclav injection is prescribed concomitantly with an aminoglycoside, the antibiotics should not be mixed in the syringe, IV fluid or giving set because of loss of activity of the aminoglycoside under these conditions.
Storage
Tablet: Store in a dry place at temperatures not exceeding 25°C.
Powder for injection: Store at temperatures not exceeding 25°C. Protect from light.
Prepared solution should be used immediately.
Powder for injection: Store at temperatures not exceeding 25°C. Protect from light.
Prepared solution should be used immediately.
Action
Antibacterial (Penicillin) + Beta-Lactamase Inhibitor.
Pharmacology: Pharmacodynamics: Amoxicillin (an aminopenicillin antibiotic) and potassium clavulanate (a β-lactamase inhibitor) (Co-amoxiclav) is usually bactericidal in action. Concurrent administration of clavulanic acid does not alter the mechanism of action of amoxicillin. However, because clavulanic acid has a high affinity for and binds to certain β-lactamases that generally inactivate amoxicillin by hydrolyzing its β-lactam ring, concurrent administration of the drug with amoxicillin results in synergistic bactericidal effect which expands amoxicillin's spectrum of activity against many strains of β-lactamase-producing bacteria resistant to amoxicillin alone.
Pharmacokinetics: Tablet: Co-amoxiclav is stable in the presence of acidic gastric secretions and is well absorbed following oral administration.
In a single-dose study in healthy male and female subjects, 19 to 44 years old, oral administration of Co-amoxiclav 1 g tablet resulted in mean peak serum amoxicillin and clavulanic acid concentrations (Cmax) of 7.787 mcg/mL and 3.078 mcg/mL, respectively. Peak serum concentrations of amoxicillin and of clavulanic acid were generally attained within 1 to 2.5 hours after oral administration. The area under the plasma concentration time curve (AUC0-t) for amoxicillin and clavulanic acid were 30.533 mcg/mL·hr and 7.184 mcg/mL·hr, respectively, while AUC0-∞ were 31.615 mcg/mL·hr and 7.487 mcg/mL·hr, respectively.
When a single oral dose of Co-amoxiclav 625 mg tablet was administered in adult subjects (fasted state), pharmacokinetic parameters reached were: Cmax 7.03 mcg/mL, AUC0-t 19.49 mcg/mL·hr, AUC0-∞ 20.91 mcg/mL·hr, and half-life (t½) 1.04 hours for amoxicillin, and Cmax 2.143 mcg/mL, AUC0-t 4.064 mcg/mL·hr, AUC0-∞ 4.722 mcg/mL·hr and t½ 1.2 hours for clavulanic acid.
Studies in healthy adults using Co-amoxiclav indicate that presence of food in the gastrointestinal tract does not affect oral absorption of either amoxicillin or clavulanic acid.
Therapeutic concentrations of both amoxicillin and clavulanic acid have been found in the gall bladder, abdominal tissue, skin, fat, and muscle tissues; the synovial and peritoneal fluids, bile and pus. Animal studies show no evidence that either component may accumulate in any organ.
Neither amoxicillin nor clavulanic acid is highly protein-bound; studies show that about 13% to 25% of total plasma drug concentration of each compound is protein-bound.
Both Co-amoxiclav components readily cross the placenta. Only small amounts of amoxicillin and clavulanic acid are distributed in human milk.
Serum concentrations of amoxicillin and clavulanic acid both decline in a biphasic manner and their t½ are similar. Approximately 50 to 73% of amoxicillin and 25 to 45% of clavulanic acid are excreted unchanged in urine within 6 to 8 hours following oral administration of a single dose of Co-amoxiclav in adults with normal renal function.
Powder for injection: At physiologic pH, both amoxicillin and clavulanic acid are fully dissociated in aqueous solution.
When Co-amoxiclav 600 mg (500 mg/100 mg) was given as a bolus intravenous (IV) injection in healthy subjects, the mean peak serum amoxicillin and clavulanic concentrations (Cmax) were 32.2 mcg/mL and 10.5 mcg/mL, respectively. The area under the plasma concentration time curve (AUC) for amoxicillin and clavulanic acid were 25.5 hr·mcg/mL and 9.2 hr·mcg/mL, respectively.
When Co-amoxiclav 1.2 g (1000 mg/200 mg) was given as a bolus IV injection in healthy subjects, the mean amoxicillin and clavulanic acid Cmax values were 105.4 mcg/mL and 28.5 mcg/mL, respectively. The AUC for amoxicillin and clavulanic acid were 76.3 hr·mcg/mL and 27.9 hr·mcg/mL, respectively.
After IV administration, therapeutic concentrations of both amoxicillin and clavulanic acid may be detected in the tissues and interstitial fluid. Therapeutic concentrations of both drugs have been found in the gall bladder, abdominal tissue, skin, fat, and muscle tissues; the synovial and peritoneal fluids, bile and pus. Animal studies show no evidence that either component may accumulate in any organ.
Neither amoxicillin nor clavulanic acid is highly protein-bound; about 13% to 25% of total plasma drug concentration of each compound is protein-bound.
Both Co-amoxiclav components readily cross the placenta. Only small amounts of amoxicillin and clavulanic acid are distributed in human milk.
Serum concentrations of amoxicillin and clavulanic acid both decline in a biphasic manner and their half-lives (0.9 to 1.1 hours) are similar.
As with other penicillins, amoxicillin is primarily eliminated via the kidneys, whereas clavulanate undergoes both renal and non-renal excretion. About 60% to 70% of amoxicillin and about 40% to 65% of clavulanic acid are excreted unchanged in urine during the first 6 hours after a single bolus IV administration of Co-amoxiclav 600 mg (500 mg/100 mg) or 1.2 g (1000 mg/200 mg) injection.
Amoxicillin is also partly excreted in the urine as the inactive penicilloic acid in quantities equivalent to 10% to 25% of the initial dose. Clavulanic acid is extensively metabolized in man to 2,5 dihydro-4-(2-hydroxyethyl)-5-oxo-1H-pyrrole-3-carboxylic acid and 1-amino-4-hydroxy-butan-2-one and eliminated in urine and feces as carbon dioxide in expired air.
Amoxicillin and clavulanic acid are both removed by hemodialysis.
Microbiology: Antimicrobial Spectrum of Activity: In vitro and clinical studies have demonstrated the susceptibility of the following microorganisms to Co-amoxiclav: See Tables 1, 2 and 3.
Pharmacology: Pharmacodynamics: Amoxicillin (an aminopenicillin antibiotic) and potassium clavulanate (a β-lactamase inhibitor) (Co-amoxiclav) is usually bactericidal in action. Concurrent administration of clavulanic acid does not alter the mechanism of action of amoxicillin. However, because clavulanic acid has a high affinity for and binds to certain β-lactamases that generally inactivate amoxicillin by hydrolyzing its β-lactam ring, concurrent administration of the drug with amoxicillin results in synergistic bactericidal effect which expands amoxicillin's spectrum of activity against many strains of β-lactamase-producing bacteria resistant to amoxicillin alone.
Pharmacokinetics: Tablet: Co-amoxiclav is stable in the presence of acidic gastric secretions and is well absorbed following oral administration.
In a single-dose study in healthy male and female subjects, 19 to 44 years old, oral administration of Co-amoxiclav 1 g tablet resulted in mean peak serum amoxicillin and clavulanic acid concentrations (Cmax) of 7.787 mcg/mL and 3.078 mcg/mL, respectively. Peak serum concentrations of amoxicillin and of clavulanic acid were generally attained within 1 to 2.5 hours after oral administration. The area under the plasma concentration time curve (AUC0-t) for amoxicillin and clavulanic acid were 30.533 mcg/mL·hr and 7.184 mcg/mL·hr, respectively, while AUC0-∞ were 31.615 mcg/mL·hr and 7.487 mcg/mL·hr, respectively.
When a single oral dose of Co-amoxiclav 625 mg tablet was administered in adult subjects (fasted state), pharmacokinetic parameters reached were: Cmax 7.03 mcg/mL, AUC0-t 19.49 mcg/mL·hr, AUC0-∞ 20.91 mcg/mL·hr, and half-life (t½) 1.04 hours for amoxicillin, and Cmax 2.143 mcg/mL, AUC0-t 4.064 mcg/mL·hr, AUC0-∞ 4.722 mcg/mL·hr and t½ 1.2 hours for clavulanic acid.
Studies in healthy adults using Co-amoxiclav indicate that presence of food in the gastrointestinal tract does not affect oral absorption of either amoxicillin or clavulanic acid.
Therapeutic concentrations of both amoxicillin and clavulanic acid have been found in the gall bladder, abdominal tissue, skin, fat, and muscle tissues; the synovial and peritoneal fluids, bile and pus. Animal studies show no evidence that either component may accumulate in any organ.
Neither amoxicillin nor clavulanic acid is highly protein-bound; studies show that about 13% to 25% of total plasma drug concentration of each compound is protein-bound.
Both Co-amoxiclav components readily cross the placenta. Only small amounts of amoxicillin and clavulanic acid are distributed in human milk.
Serum concentrations of amoxicillin and clavulanic acid both decline in a biphasic manner and their t½ are similar. Approximately 50 to 73% of amoxicillin and 25 to 45% of clavulanic acid are excreted unchanged in urine within 6 to 8 hours following oral administration of a single dose of Co-amoxiclav in adults with normal renal function.
Powder for injection: At physiologic pH, both amoxicillin and clavulanic acid are fully dissociated in aqueous solution.
When Co-amoxiclav 600 mg (500 mg/100 mg) was given as a bolus intravenous (IV) injection in healthy subjects, the mean peak serum amoxicillin and clavulanic concentrations (Cmax) were 32.2 mcg/mL and 10.5 mcg/mL, respectively. The area under the plasma concentration time curve (AUC) for amoxicillin and clavulanic acid were 25.5 hr·mcg/mL and 9.2 hr·mcg/mL, respectively.
When Co-amoxiclav 1.2 g (1000 mg/200 mg) was given as a bolus IV injection in healthy subjects, the mean amoxicillin and clavulanic acid Cmax values were 105.4 mcg/mL and 28.5 mcg/mL, respectively. The AUC for amoxicillin and clavulanic acid were 76.3 hr·mcg/mL and 27.9 hr·mcg/mL, respectively.
After IV administration, therapeutic concentrations of both amoxicillin and clavulanic acid may be detected in the tissues and interstitial fluid. Therapeutic concentrations of both drugs have been found in the gall bladder, abdominal tissue, skin, fat, and muscle tissues; the synovial and peritoneal fluids, bile and pus. Animal studies show no evidence that either component may accumulate in any organ.
Neither amoxicillin nor clavulanic acid is highly protein-bound; about 13% to 25% of total plasma drug concentration of each compound is protein-bound.
Both Co-amoxiclav components readily cross the placenta. Only small amounts of amoxicillin and clavulanic acid are distributed in human milk.
Serum concentrations of amoxicillin and clavulanic acid both decline in a biphasic manner and their half-lives (0.9 to 1.1 hours) are similar.
As with other penicillins, amoxicillin is primarily eliminated via the kidneys, whereas clavulanate undergoes both renal and non-renal excretion. About 60% to 70% of amoxicillin and about 40% to 65% of clavulanic acid are excreted unchanged in urine during the first 6 hours after a single bolus IV administration of Co-amoxiclav 600 mg (500 mg/100 mg) or 1.2 g (1000 mg/200 mg) injection.
Amoxicillin is also partly excreted in the urine as the inactive penicilloic acid in quantities equivalent to 10% to 25% of the initial dose. Clavulanic acid is extensively metabolized in man to 2,5 dihydro-4-(2-hydroxyethyl)-5-oxo-1H-pyrrole-3-carboxylic acid and 1-amino-4-hydroxy-butan-2-one and eliminated in urine and feces as carbon dioxide in expired air.
Amoxicillin and clavulanic acid are both removed by hemodialysis.
Microbiology: Antimicrobial Spectrum of Activity: In vitro and clinical studies have demonstrated the susceptibility of the following microorganisms to Co-amoxiclav: See Tables 1, 2 and 3.



MedsGo Class
Penicillins
Features
Dosage
1g
Ingredients
- Co-Amoxiclav
Packaging
Film-Coated Tablet 1's
Generic Name
Co-Amoxiclav
Registration Number
DRP-1059
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions

