All variations
Indications/Uses
Ketesse FC tab: Treatment of pain of mild to moderate intensity, like musculo-skeletal, dysmenorrheal, dental pain.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Symptomatic treatment of acute pain of moderate to severe intensity, when oral administration is not appropriate such as post-operative pain, renal colic and low back pain.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Symptomatic treatment of acute pain of moderate to severe intensity, when oral administration is not appropriate such as post-operative pain, renal colic and low back pain.
Dosage/Direction for Use
Ketesse FC tab: General Population: According to the nature and severity of pain, the recommended dosage is generally 12.5 mg every 4-6 hours or 25 mg every 8 hours. The total daily dose should not exceed 75 mg. Undesirable effects may be minimized by using the lowest effective dose for the shortest duration necessary to control symptoms.
Dexketoprofen (KETESSE) tablets are not intended for long-term use and the treatment must be limited to the symptomatic period.
Concomitant administration with food delays the absorption rate of the drug (see Pharmacology: Pharmacokinetics under Actions), thus in case of acute pain, it is recommended that administration is at least 30 min before meals.
Elderly: In elderly patients it is recommended to start the therapy at the lower end of the dosage range (50 mg total daily dose). The dosage may be increased to that recommended for the general population only after good general tolerance has been ascertained.
Hepatic Dysfunction: Patients with mild to moderate hepatic dysfunction should start therapy at reduced doses (50 mg total daily dose) and be closely monitored. Dexketoprofen (KETESSE) tablets should not be used in patients with severe hepatic dysfunction.
Renal Dysfunction: The initial dosage should be reduced to 50 mg total daily dose in patients with mildly impaired renal function. Dexketoprofen (KETESSE) tablets should not be used in patients with moderate to severe renal dysfunction (see Contraindications).
Children and adolescents: Dexketoprofen (KETESSE) tablets has not been studied in children and adolescent. Therefore, safety and efficacy have not been established and the product should not be used in children and adolescent.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Adults: The recommended dose is 50 mg every 8-12 hours. If necessary, the administration can be repeated 6 hours apart. The total daily dose should not exceed 150 mg.
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion is intended for short term use and the treatment must be limited to the acute symptomatic period (no more than two days). Patients should be switched to an oral analgesic treatment when possible.
Undesirable effects may be minimized by using the lowest effective dose for the shortest duration necessary to control symptoms.
In case of moderate to severe postoperative pain, Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion can be used in combination with opioid analgesics, if indicated, at the same recommended doses in adults.
Elderly: No dosage adjustment is generally necessary in elderly patients. However because of the physiological decline in renal function in elderly patients a lower dose is recommended in case of mild renal function impairment: 50 mg total daily dose.
Liver Disease: The dosage should be reduced to 50 mg total daily dose in patients with mild to moderate (Child-Pugh score 5 - 9) hepatic impairment and hepatic function should be closely monitored. Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should not be used in patients with severe hepatic dysfunction (Child-Pugh score 10 - 15).
Renal dysfunction: The dosage should be reduced to 50 mg total daily dose in patients with mildly impaired renal function (creatinine clearance 50 - 80 mL/min). Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should not be used in patients with moderate to severe renal dysfunction (creatinine clearance <50 mL/min).
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should not be used in patients with moderate to severe renal dysfunction (creatinine clearance <50 mL/min).
Children and adolescents: Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion has not been studied in children and adolescents. Therefore, safety and efficacy have not been established and the product should not be used in children and adolescents.
Administration: Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion can be administered either by intramuscular or by intravenous route: Intramuscular use: The content of one ampoule (2 mL) of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should be administered by slow Injection deep into the muscle.
Intravenous use: Intravenous infusion: the diluted solution, should be administered as a slow intravenous infusion, lasting 10 to 30 min. The solution must be always protected from natural daylight.
Intravenous bolus: if necessary, the content of one ampoule (2 mL) of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion can be administered in a slow intravenous bolus over no less than 15 seconds.
Instructions on handling the product: When Dexketoprofen (KETESSE) is administered intramuscularly or as intravenous bolus, the solution should be injected immediately after its removal from the coloured ampoule. For administration as intravenous infusion, the solution should be diluted aseptically and protected from natural daylight.
Dexketoprofen (KETESSE) tablets are not intended for long-term use and the treatment must be limited to the symptomatic period.
Concomitant administration with food delays the absorption rate of the drug (see Pharmacology: Pharmacokinetics under Actions), thus in case of acute pain, it is recommended that administration is at least 30 min before meals.
Elderly: In elderly patients it is recommended to start the therapy at the lower end of the dosage range (50 mg total daily dose). The dosage may be increased to that recommended for the general population only after good general tolerance has been ascertained.
Hepatic Dysfunction: Patients with mild to moderate hepatic dysfunction should start therapy at reduced doses (50 mg total daily dose) and be closely monitored. Dexketoprofen (KETESSE) tablets should not be used in patients with severe hepatic dysfunction.
Renal Dysfunction: The initial dosage should be reduced to 50 mg total daily dose in patients with mildly impaired renal function. Dexketoprofen (KETESSE) tablets should not be used in patients with moderate to severe renal dysfunction (see Contraindications).
Children and adolescents: Dexketoprofen (KETESSE) tablets has not been studied in children and adolescent. Therefore, safety and efficacy have not been established and the product should not be used in children and adolescent.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Adults: The recommended dose is 50 mg every 8-12 hours. If necessary, the administration can be repeated 6 hours apart. The total daily dose should not exceed 150 mg.
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion is intended for short term use and the treatment must be limited to the acute symptomatic period (no more than two days). Patients should be switched to an oral analgesic treatment when possible.
Undesirable effects may be minimized by using the lowest effective dose for the shortest duration necessary to control symptoms.
In case of moderate to severe postoperative pain, Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion can be used in combination with opioid analgesics, if indicated, at the same recommended doses in adults.
Elderly: No dosage adjustment is generally necessary in elderly patients. However because of the physiological decline in renal function in elderly patients a lower dose is recommended in case of mild renal function impairment: 50 mg total daily dose.
Liver Disease: The dosage should be reduced to 50 mg total daily dose in patients with mild to moderate (Child-Pugh score 5 - 9) hepatic impairment and hepatic function should be closely monitored. Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should not be used in patients with severe hepatic dysfunction (Child-Pugh score 10 - 15).
Renal dysfunction: The dosage should be reduced to 50 mg total daily dose in patients with mildly impaired renal function (creatinine clearance 50 - 80 mL/min). Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should not be used in patients with moderate to severe renal dysfunction (creatinine clearance <50 mL/min).
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should not be used in patients with moderate to severe renal dysfunction (creatinine clearance <50 mL/min).
Children and adolescents: Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion has not been studied in children and adolescents. Therefore, safety and efficacy have not been established and the product should not be used in children and adolescents.
Administration: Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion can be administered either by intramuscular or by intravenous route: Intramuscular use: The content of one ampoule (2 mL) of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should be administered by slow Injection deep into the muscle.
Intravenous use: Intravenous infusion: the diluted solution, should be administered as a slow intravenous infusion, lasting 10 to 30 min. The solution must be always protected from natural daylight.
Intravenous bolus: if necessary, the content of one ampoule (2 mL) of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion can be administered in a slow intravenous bolus over no less than 15 seconds.
Instructions on handling the product: When Dexketoprofen (KETESSE) is administered intramuscularly or as intravenous bolus, the solution should be injected immediately after its removal from the coloured ampoule. For administration as intravenous infusion, the solution should be diluted aseptically and protected from natural daylight.
Overdosage
In case of accidental or excessive intake or administration, immediately institute symptomatic therapy according to the patient's clinical condition. Dexketoprofen trometamol may be removed by dialysis.
Ketesse FC tab: Activated charcoal should be administered if more than 5 mg/kg has been ingested by an adult or a child within an hour.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.):The symptomatology following overdose is not known. Similar medicinal products have produced gastrointestinal (vomiting, anorexia, abdominal pain) and neurological (somnolence, vertigo, disorientation, headache) disorders.
Ketesse FC tab: Activated charcoal should be administered if more than 5 mg/kg has been ingested by an adult or a child within an hour.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.):The symptomatology following overdose is not known. Similar medicinal products have produced gastrointestinal (vomiting, anorexia, abdominal pain) and neurological (somnolence, vertigo, disorientation, headache) disorders.
Administration
Should be taken on an empty stomach: Take 30 min before meals, especially for quick relief of acute pain.
Contraindications
Absolute Contraindications: Not to be given to those patients who have history of: Stroke: cerebrovascular accident, CVA.
Heart attack: Myocardial infarction, MI.
Coronary artery bypass graft: CABG.
Uncontrolled hypertension.
Congestive heart failure (CHF) NYHA II-IV.
Ketesse FC tab: Dexketoprofen (KETESSE) tablets must not be administered in the following cases: patients hypersensitive to Dexketoprofen, to any other NSAID, or to any of the excipients of the product; patients in whom substances with a similar action (e.g. aspirin, or other NSAIDs) precipitate attacks of asthma, bronchospasm, acute rhinitis, or cause nasal polyps, urticaria or angioneurotic edema; patients with active or suspected peptic ulcer/haemorrhage or history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding) or chronic dyspepsia; patients with history of gastrointestinal bleeding or perforation, related to previous NSAIDs therapy; patients who have gastrointestinal bleeding or other active bleedings or bleeding disorders; patients with Crohn's disease or ulcerative colitis; patients with a history of bronchial asthma; patients with severe heart failure; patients with moderate to severe renal dysfunction; patients with severely impaired hepatic function; patients with haemorrhagic diathesis and other coagulation disorders; during the third trimester of pregnancy and the lactation period.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V/I.M.): Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion must not be administered in the following cases: patients hypersensitive to Dexketoprofen, to any other NSAID, or to any of the excipients of this medicinal product; patients in whom substances with a similar action (e.g. acetylsalicylic acid and other NSAIDs) precipitate attacks of asthma, bronchospasm, acute rhinitis, or cause nasal polyps, urticaria or angioneurotic edema; patients with active or suspected peptic ulcer/haemorrhage or history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding); patients who have gastrointestinal bleeding or other active bleedings or bleeding disorders; patients with history of gastrointestinal bleeding or perforation, related to previous NSAIDs therapy; patients with Crohn's disease or ulcerative colitis; patients with a history of bronchial asthma; patients with severe heart failure; patients with moderate to severe renal dysfunction (creatinine clearance <50 mL/min); patients with severely impaired hepatic function (Child-Pugh score 10-15); patients with haemorrhagic diathesis and other coagulation disorders; during the third trimester of pregnancy and the lactation period.
Decketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion is contraindicated for neuraxial (intrathecal or epidural) administration due to its ethanol content.
Heart attack: Myocardial infarction, MI.
Coronary artery bypass graft: CABG.
Uncontrolled hypertension.
Congestive heart failure (CHF) NYHA II-IV.
Ketesse FC tab: Dexketoprofen (KETESSE) tablets must not be administered in the following cases: patients hypersensitive to Dexketoprofen, to any other NSAID, or to any of the excipients of the product; patients in whom substances with a similar action (e.g. aspirin, or other NSAIDs) precipitate attacks of asthma, bronchospasm, acute rhinitis, or cause nasal polyps, urticaria or angioneurotic edema; patients with active or suspected peptic ulcer/haemorrhage or history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding) or chronic dyspepsia; patients with history of gastrointestinal bleeding or perforation, related to previous NSAIDs therapy; patients who have gastrointestinal bleeding or other active bleedings or bleeding disorders; patients with Crohn's disease or ulcerative colitis; patients with a history of bronchial asthma; patients with severe heart failure; patients with moderate to severe renal dysfunction; patients with severely impaired hepatic function; patients with haemorrhagic diathesis and other coagulation disorders; during the third trimester of pregnancy and the lactation period.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V/I.M.): Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion must not be administered in the following cases: patients hypersensitive to Dexketoprofen, to any other NSAID, or to any of the excipients of this medicinal product; patients in whom substances with a similar action (e.g. acetylsalicylic acid and other NSAIDs) precipitate attacks of asthma, bronchospasm, acute rhinitis, or cause nasal polyps, urticaria or angioneurotic edema; patients with active or suspected peptic ulcer/haemorrhage or history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding); patients who have gastrointestinal bleeding or other active bleedings or bleeding disorders; patients with history of gastrointestinal bleeding or perforation, related to previous NSAIDs therapy; patients with Crohn's disease or ulcerative colitis; patients with a history of bronchial asthma; patients with severe heart failure; patients with moderate to severe renal dysfunction (creatinine clearance <50 mL/min); patients with severely impaired hepatic function (Child-Pugh score 10-15); patients with haemorrhagic diathesis and other coagulation disorders; during the third trimester of pregnancy and the lactation period.
Decketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion is contraindicated for neuraxial (intrathecal or epidural) administration due to its ethanol content.
Special Precautions
Ketesse FC tab: The safe use in children and adolescents has not been established.
Administer with caution in patients with a history of allergic conditions.
The use of Dexketoprofen (KETESSE) with concomitant other NSAIDs including cyclooxygenase-2 selective inhibitors should be avoided.
Undesirable effects may be minimized by using the minimum effective dose for the shortest duration necessary to control symptoms.
Gastrointestinal bleeding, ulceration or perforation which can be fatal, have been reported with all NSAIDs at anytime during treatment, with or without warning symptoms or a previous history of serious gastrointestinal events. When gastrointestinal bleeding or ulceration occurs in patients receiving Dexketoprofen (KETESSE), the treatment should be withdrawn.
The risk of gastrointestinal bleeding, ulceration or perforation is higher with increasing NSAID doses, in patients with a history of ulcer, particularly if complicated with haemorrhage or perforation and in the elderly.
As with all NSAIDs, any history of esophagitis, gastritis and/or peptic ulcer must be sought in order to ensure their total cure before starting treatment with Dexketoprofen trometamol. Patients with gastrointestinal symptoms or history of gastrointestinal disease should be monitored for digestive disturbances, especially gastrointestinal bleeding.
NSAIDs should be given with care to patients with a history of gastrointestinal disease (ulcerative colitis, Crohn's disease) as their condition may be exacerbated.
Combination therapy with protective agents (e.g. misoprostol or proton pump inhibitors) should be considered for these patients, and also for patients requiring concomitant low dose aspirin, or other drugs likely to increase gastrointestinal risk.
Patients with a history of gastrointestinal toxicity, particularly when elderly, should report any unusual abdominal symptoms (especially gastrointestinal bleeding) particularly in the initial stages of treatment.
Caution should be advised in patients receiving concomitant medications which could increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin-reuptake inhibitors or anti-platelet agents such as aspirin.
All non-selective NSAIDs can inhibit platelet aggregation and prolong bleeding time via inhibition of prostaglandin synthesis. Therefore, the use of Dexketoprofen trometamol in patients who are receiving other therapy that interferes with haemostasis, such as warfarin or other coumarins or heparins is not recommended.
As with all NSAIDs, it can increase plasma urea nitrogen and creatinine. As with other inhibitors of prostaglandin synthesis, it can be associated with adverse effects on the renal system which can lead to glomerular nephritis, interstitial nephritis, renal papillary necrosis, nephrotic syndrome and acute renal failure.
As with other NSAIDs, it can cause transient small increases in some liver parameters, and also significant increases in SGOT and SGPT. In case of a relevant increase in such parameters, therapy must be discontinued.
Dexketoprofen (KETESSE) tablets should be administered with caution to patients suffering from haematopoietic disorders, systemic lupus erythematosus or mixed connective tissue disease. As other NSAIDs, Dexketoprofen can mask the symptoms of infectious diseases.
Caution should be exercised in patients with impairment of hepatic and/or renal functions as well as in patients with a history of hypertension and/or heart failure. In these patients, the use of NSAIDs may result in deterioration of renal function, fluid retention and edema. Caution is also required in patients receiving diuretic therapy or those who could develop hypovolaemia as there is an increased risk of nephrotoxicity. Special caution should be exercised in patients with a history of cardiac disease, in particular those with previous episodes of heart failure as there is an increased risk of triggering heart failure.
Elderly patients are more likely to be suffering from impaired renal cardiovascular or hepatic function.
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs. Patients appear to be at highest risk of these reactions early in the course of therapy, the onset of the reaction occurring in the majority of cases within the first month of treatment. Dexketoprofen (KETESSE) should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity.
As with other NSAIDs, the use of Dexketoprofen trometamol may impair female fertility and is not recommended in women attempting to conceive. In women who have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of Dexketoprofen trometamol should be considered. Appropriate monitoring and advice are required for patients with a history of hypertension and/or mild to moderate congestive heart failure as fluid retention and edema have been reported in association with NSAIDs therapy.
Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long term treatment) may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke). There are insufficient data to exclude such a risk for Dexketoprofen trometamol.
Patients with uncontrolled hypertension, congestive heart failure, established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease should only be treated with Dexketoprofen trometamol after careful consideration. Similar consideration should be made before initiating longer-term treatment of the patients with risk factors for cardiovascular disease (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking).
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): The safe use in children and adolescents has not been established.
Administer with caution in patients with a history of allergic conditions.
The use of Dexketoprofen (KETESSE) with concomitant NSAIDs including cyclooxygenase-2 selective inhibitors should be avoided.
Undesirable effects may be minimized by using the lowest effective dose for the shortest duration necessary to control symptoms.
Gastrointestinal bleeding, ulceration and perforation: gastrointestinal bleeding, ulceration or perforation, which can be fatal, has been reported with all NSAIDs at anytime during treatment, with or without warning symptoms or a previous history of serious gastrointestinal events. When gastrointestinal bleeding or ulceration occurs in patients receiving dexketoprofen, the treatment should be withdrawn.
The risk of gastrointestinal bleeding, ulceration or perforation is higher with increasing NSAID doses, in patients with a history of ulcer, particularly if complicated with haemorrhage or perforation, and in the elderly.
NSAIDs should be given with care to patients with a history of gastrointestinal disease (ulcerative colitis, Crohn’s disease) as these condition may be exacerbated.
As with all NSAIDs, any history of oesophagitis, gastritis and/or peptic ulcer must be sought in order to ensure their total cure before starting treatment with Dexketoprofen trometamol. Patients with gastrointestinal symptoms or history of gastrointestinal disease should be monitored for digestive disturbances, especially gastrointestinal bleeding.
Combination therapy with protective agents (e.g. misoprostol or proton pump inhibitors) should be considered for these patients, and also for patients requiring concomitant low dose aspirin, or other drugs likely to increase gastrointestinal risk.
Patients with a history of gastrointestinal toxicity, particularly when elderly, should report any unusual abdominal symptoms (especially gastrointestinal bleeding) particularly in the initial stages of treatment.
Caution should be advised in patients receiving concomitant medications which could increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin-reuptake inhibitors or anti-platelet agents such as aspirin.
All non-selective NSAIDs can inhibit platelet aggregation and prolong bleeding time via inhibition of prostaglandin synthesis. The concomitant use of dexketoprofen trometamol and prophylactic doses of low molecular weight heparin in the postoperative period has been assessed in controlled clinical trials and no effect on coagulation parameters was observed. Nevertheless, patients who are receiving therapy that interferes with haemostasis, such as warfarin or other coumarins or heparins should be carefully monitored if dexketoprofen trometamol is administered.
Appropriate monitoring and advice are required for patients with a history of hypertension and/or mild to moderate congestive heart failure as fluid retention and/or edema have been reported in association with NSAID therapy.
Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long term treatment) may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke). There are insufficient data to exclude such a risk for Dexketoprofen Trometamol.
Patients with uncontrolled hypertension, congestive heart failure, established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease should only be treated with Dexketoprofen Trometamol after careful consideration. Similar consideration should be made before initiating longer-term treatment of patients with risk factors for cardiovascular disease (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking).
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs. Patients appear to be at highest risk for these reactions early in the course of therapy: the onset of the reaction occurring in the majority of cases within the first month of treatment. Dexketoprofen (KETESSE) should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity.
As with all NSAIDs, it can increase plasma urea nitrogen and creatinine. As with other inhibitors of prostaglandin synthesis, it can be associated with adverse effects on the renal system, which can lead to glomerular nephritis, interstitial nephritis, renal papillary necrosis, nephrotic syndrome and acute renal failure.
As with other NSAIDs, it can cause transient small increases in some liver parameters, and also significant increases in SGOT and SGPT. In case of a relevant increase in such parameters, therapy must be discontinued.
Caution should be exercised in patients with impairment of hepatic and/or renal functions as well as in patients with a history of hypertension and/or heart failure. In these patients, the use of NSAIDs may result in deterioration of renal function, fluid retention and edema.
Caution is also required in patients receiving diuretic therapy or those who could develop hypovolaemia as there is an increased risk of nephrotoxicity. Special caution should be exercised in patients with a history of cardiac disease, in particular those with previous episodes of heart failure as there is an increased risk of triggering heart failure.
Elderly patients are more likely to be suffering from impaired renal cardiovascular or hepatic function.
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should be administered with caution to patients suffering from haematopoietic disorders, systemic lupus erythematosus or mixed connective tissue disease.
As other NSAIDs, dexketoprofen can mask the symptoms of infectious diseases. In isolated cases an aggravation of soft tissue infections has been described in temporal connection with the use of NSAIDs. Therefore the patient is advised to consult a physician immediately if signs of a bacterial infection occur or worsen during therapy.
As with other NSAIDs, the use of dexketoprofen trometamol may impair female fertility and is not recommended in women attempting to conceive. In women who have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of dexketoprofen trometamol should be considered. Dexketoprofen should not be used during first and second trimester of pregnancy unless clearly necessary.
Each ampoule of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion contains 200 mg of ethanol equivalent to 5 mL beer or 2.08 mL wine per dose.
Harmful for those suffering from alcoholism.
To be taken into account in pregnant and breast-feeding women, children and high-risk groups such as patients with liver disease, or epilepsy.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially "sodium-free".
Effects on the ability to drive and use machines: Ketesse FC tab and Ketesse for Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Dexketoprofen (KETESSE) tablets can cause minor or moderate effects/influence on the ability to drive or use machines due to the possibility of dizziness or drowsiness occurring.
Use in elderly: The elderly have an increased frequency of adverse reactions to NSAIDs especially gastrointestinal bleeding and perforation which may be fatal.
These patients should commence treatment on the lowest dose available.
Administer with caution in patients with a history of allergic conditions.
The use of Dexketoprofen (KETESSE) with concomitant other NSAIDs including cyclooxygenase-2 selective inhibitors should be avoided.
Undesirable effects may be minimized by using the minimum effective dose for the shortest duration necessary to control symptoms.
Gastrointestinal bleeding, ulceration or perforation which can be fatal, have been reported with all NSAIDs at anytime during treatment, with or without warning symptoms or a previous history of serious gastrointestinal events. When gastrointestinal bleeding or ulceration occurs in patients receiving Dexketoprofen (KETESSE), the treatment should be withdrawn.
The risk of gastrointestinal bleeding, ulceration or perforation is higher with increasing NSAID doses, in patients with a history of ulcer, particularly if complicated with haemorrhage or perforation and in the elderly.
As with all NSAIDs, any history of esophagitis, gastritis and/or peptic ulcer must be sought in order to ensure their total cure before starting treatment with Dexketoprofen trometamol. Patients with gastrointestinal symptoms or history of gastrointestinal disease should be monitored for digestive disturbances, especially gastrointestinal bleeding.
NSAIDs should be given with care to patients with a history of gastrointestinal disease (ulcerative colitis, Crohn's disease) as their condition may be exacerbated.
Combination therapy with protective agents (e.g. misoprostol or proton pump inhibitors) should be considered for these patients, and also for patients requiring concomitant low dose aspirin, or other drugs likely to increase gastrointestinal risk.
Patients with a history of gastrointestinal toxicity, particularly when elderly, should report any unusual abdominal symptoms (especially gastrointestinal bleeding) particularly in the initial stages of treatment.
Caution should be advised in patients receiving concomitant medications which could increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin-reuptake inhibitors or anti-platelet agents such as aspirin.
All non-selective NSAIDs can inhibit platelet aggregation and prolong bleeding time via inhibition of prostaglandin synthesis. Therefore, the use of Dexketoprofen trometamol in patients who are receiving other therapy that interferes with haemostasis, such as warfarin or other coumarins or heparins is not recommended.
As with all NSAIDs, it can increase plasma urea nitrogen and creatinine. As with other inhibitors of prostaglandin synthesis, it can be associated with adverse effects on the renal system which can lead to glomerular nephritis, interstitial nephritis, renal papillary necrosis, nephrotic syndrome and acute renal failure.
As with other NSAIDs, it can cause transient small increases in some liver parameters, and also significant increases in SGOT and SGPT. In case of a relevant increase in such parameters, therapy must be discontinued.
Dexketoprofen (KETESSE) tablets should be administered with caution to patients suffering from haematopoietic disorders, systemic lupus erythematosus or mixed connective tissue disease. As other NSAIDs, Dexketoprofen can mask the symptoms of infectious diseases.
Caution should be exercised in patients with impairment of hepatic and/or renal functions as well as in patients with a history of hypertension and/or heart failure. In these patients, the use of NSAIDs may result in deterioration of renal function, fluid retention and edema. Caution is also required in patients receiving diuretic therapy or those who could develop hypovolaemia as there is an increased risk of nephrotoxicity. Special caution should be exercised in patients with a history of cardiac disease, in particular those with previous episodes of heart failure as there is an increased risk of triggering heart failure.
Elderly patients are more likely to be suffering from impaired renal cardiovascular or hepatic function.
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs. Patients appear to be at highest risk of these reactions early in the course of therapy, the onset of the reaction occurring in the majority of cases within the first month of treatment. Dexketoprofen (KETESSE) should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity.
As with other NSAIDs, the use of Dexketoprofen trometamol may impair female fertility and is not recommended in women attempting to conceive. In women who have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of Dexketoprofen trometamol should be considered. Appropriate monitoring and advice are required for patients with a history of hypertension and/or mild to moderate congestive heart failure as fluid retention and edema have been reported in association with NSAIDs therapy.
Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long term treatment) may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke). There are insufficient data to exclude such a risk for Dexketoprofen trometamol.
Patients with uncontrolled hypertension, congestive heart failure, established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease should only be treated with Dexketoprofen trometamol after careful consideration. Similar consideration should be made before initiating longer-term treatment of the patients with risk factors for cardiovascular disease (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking).
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): The safe use in children and adolescents has not been established.
Administer with caution in patients with a history of allergic conditions.
The use of Dexketoprofen (KETESSE) with concomitant NSAIDs including cyclooxygenase-2 selective inhibitors should be avoided.
Undesirable effects may be minimized by using the lowest effective dose for the shortest duration necessary to control symptoms.
Gastrointestinal bleeding, ulceration and perforation: gastrointestinal bleeding, ulceration or perforation, which can be fatal, has been reported with all NSAIDs at anytime during treatment, with or without warning symptoms or a previous history of serious gastrointestinal events. When gastrointestinal bleeding or ulceration occurs in patients receiving dexketoprofen, the treatment should be withdrawn.
The risk of gastrointestinal bleeding, ulceration or perforation is higher with increasing NSAID doses, in patients with a history of ulcer, particularly if complicated with haemorrhage or perforation, and in the elderly.
NSAIDs should be given with care to patients with a history of gastrointestinal disease (ulcerative colitis, Crohn’s disease) as these condition may be exacerbated.
As with all NSAIDs, any history of oesophagitis, gastritis and/or peptic ulcer must be sought in order to ensure their total cure before starting treatment with Dexketoprofen trometamol. Patients with gastrointestinal symptoms or history of gastrointestinal disease should be monitored for digestive disturbances, especially gastrointestinal bleeding.
Combination therapy with protective agents (e.g. misoprostol or proton pump inhibitors) should be considered for these patients, and also for patients requiring concomitant low dose aspirin, or other drugs likely to increase gastrointestinal risk.
Patients with a history of gastrointestinal toxicity, particularly when elderly, should report any unusual abdominal symptoms (especially gastrointestinal bleeding) particularly in the initial stages of treatment.
Caution should be advised in patients receiving concomitant medications which could increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin-reuptake inhibitors or anti-platelet agents such as aspirin.
All non-selective NSAIDs can inhibit platelet aggregation and prolong bleeding time via inhibition of prostaglandin synthesis. The concomitant use of dexketoprofen trometamol and prophylactic doses of low molecular weight heparin in the postoperative period has been assessed in controlled clinical trials and no effect on coagulation parameters was observed. Nevertheless, patients who are receiving therapy that interferes with haemostasis, such as warfarin or other coumarins or heparins should be carefully monitored if dexketoprofen trometamol is administered.
Appropriate monitoring and advice are required for patients with a history of hypertension and/or mild to moderate congestive heart failure as fluid retention and/or edema have been reported in association with NSAID therapy.
Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long term treatment) may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke). There are insufficient data to exclude such a risk for Dexketoprofen Trometamol.
Patients with uncontrolled hypertension, congestive heart failure, established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease should only be treated with Dexketoprofen Trometamol after careful consideration. Similar consideration should be made before initiating longer-term treatment of patients with risk factors for cardiovascular disease (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking).
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs. Patients appear to be at highest risk for these reactions early in the course of therapy: the onset of the reaction occurring in the majority of cases within the first month of treatment. Dexketoprofen (KETESSE) should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity.
As with all NSAIDs, it can increase plasma urea nitrogen and creatinine. As with other inhibitors of prostaglandin synthesis, it can be associated with adverse effects on the renal system, which can lead to glomerular nephritis, interstitial nephritis, renal papillary necrosis, nephrotic syndrome and acute renal failure.
As with other NSAIDs, it can cause transient small increases in some liver parameters, and also significant increases in SGOT and SGPT. In case of a relevant increase in such parameters, therapy must be discontinued.
Caution should be exercised in patients with impairment of hepatic and/or renal functions as well as in patients with a history of hypertension and/or heart failure. In these patients, the use of NSAIDs may result in deterioration of renal function, fluid retention and edema.
Caution is also required in patients receiving diuretic therapy or those who could develop hypovolaemia as there is an increased risk of nephrotoxicity. Special caution should be exercised in patients with a history of cardiac disease, in particular those with previous episodes of heart failure as there is an increased risk of triggering heart failure.
Elderly patients are more likely to be suffering from impaired renal cardiovascular or hepatic function.
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should be administered with caution to patients suffering from haematopoietic disorders, systemic lupus erythematosus or mixed connective tissue disease.
As other NSAIDs, dexketoprofen can mask the symptoms of infectious diseases. In isolated cases an aggravation of soft tissue infections has been described in temporal connection with the use of NSAIDs. Therefore the patient is advised to consult a physician immediately if signs of a bacterial infection occur or worsen during therapy.
As with other NSAIDs, the use of dexketoprofen trometamol may impair female fertility and is not recommended in women attempting to conceive. In women who have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of dexketoprofen trometamol should be considered. Dexketoprofen should not be used during first and second trimester of pregnancy unless clearly necessary.
Each ampoule of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion contains 200 mg of ethanol equivalent to 5 mL beer or 2.08 mL wine per dose.
Harmful for those suffering from alcoholism.
To be taken into account in pregnant and breast-feeding women, children and high-risk groups such as patients with liver disease, or epilepsy.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially "sodium-free".
Effects on the ability to drive and use machines: Ketesse FC tab and Ketesse for Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Dexketoprofen (KETESSE) tablets can cause minor or moderate effects/influence on the ability to drive or use machines due to the possibility of dizziness or drowsiness occurring.
Use in elderly: The elderly have an increased frequency of adverse reactions to NSAIDs especially gastrointestinal bleeding and perforation which may be fatal.
These patients should commence treatment on the lowest dose available.
Use In Pregnancy & Lactation
Use in Pregnancy: Inhibition of prostaglandin synthesis may adversely affect the pregnancy and/or the embryo/foetal development. Data from epidemiological studies raise concern about an increased risk of miscarriage and of cardiac malformation and gastroschisis after use of a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk for cardiovascular malformation was increased from less than 1%, up to approximately 1.5%. The risk is believed to increase with dose and duration of therapy. In animals, administration of a prostaglandin synthesis inhibitor has been shown to result in increased pre- and post-implantation loss and embryo-foetal lethality. In addition, increased incidences of various malformations including cardiovascular, have been reported in animals given a prostaglandin synthesis inhibitor during the organogenetic period. Nevertheless, animal studies with dexketoprofen trometamol haven't shown reproductive toxicity. During the first and second trimester of pregnancy, Dexketoprofen trometamol should not be given unless clearly necessary. If Dexketoprofen trometamol is used by a women attempting to conceive, or during the first and second trimester of pregnancy, the dose should be kept as low and duration of treatment as short as possible.
During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the fetus to: cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension); renal dysfunction, which may progress to renal failure with oligo-hydroamniosis.
At the end of pregnancy, all prostaglandin synthesis inhibitors may expose the mother and the neonate to: possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses; inhibition of uterine contractions resulting in delayed or prolonged labour. It is not known whether dexketoprofen is excreted in human milk.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion is contraindicated during third trimester of pregnancy and lactation.
During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the fetus to: cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension); renal dysfunction, which may progress to renal failure with oligo-hydroamniosis.
At the end of pregnancy, all prostaglandin synthesis inhibitors may expose the mother and the neonate to: possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses; inhibition of uterine contractions resulting in delayed or prolonged labour. It is not known whether dexketoprofen is excreted in human milk.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion is contraindicated during third trimester of pregnancy and lactation.
Adverse Reactions
The adverse reactions reported as at least possibly related with dexketoprofen trometamol in clinical trials, as well the adverse reactions reported after the marketing of Ketesse tablets and solution for injection or concentrate for solution for infusion are tabulated as follows, classified by system organ class and ordered by frequency: (See Table 1 and Table 2.)
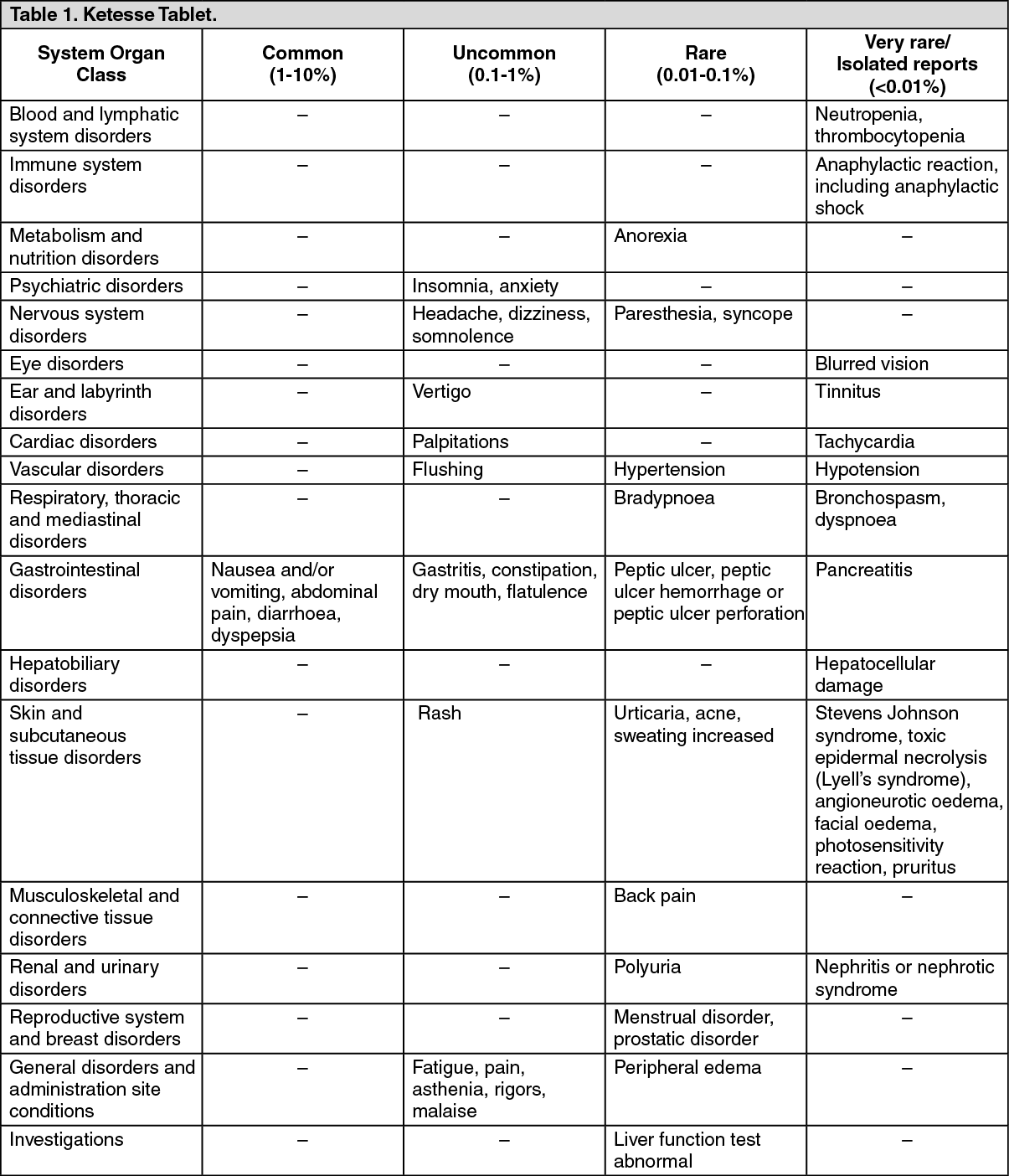

Gastrointestinal: The most commonly observed adverse events are gastrointestinal in nature. Peptic ulcers, perforation or gastrointestinal bleeding, sometimes fatal, particularly in the elderly, may occur. Nausea, vomiting, diarrhoea, flatulence, constipation, dyspepsia, abdominal pain, melaena, haematemesis, ulcerative stomatitis, exacerbation of colitis and Crohn's disease have been reported following administration. Less frequently, gastritis has been observed. Edema, hypertension and cardiac failure have been reported in association with NSAIDs treatment.
As with other NSAIDs the following undesirable effects may appear: Aseptic meningitis, which might predominantly occur in patients with systemic lupus erythematosus or mixed connective tissue disease; hematological reactions (purpura, aplastic and hemolytic anemia and rarely agranulocytosis and medullar hypoplasia).
Bullous reactions including Stevens-Johnson syndrome and Toxic Epidermal Necrolysis (very rare).
Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long-term treatment) may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke).
Drug Interactions
The following interactions apply to non-steroidal anti-inflammatory drugs (NSAIDs) in general: Inadvisable combinations: Other NSAIDs, including high doses of salicylates ≥3 g/day): administration of several NSAIDs together may increase the risk of gastrointestinal ulcers and bleeding, via a synergistic effect.
Anticoagulants: NSAIDs may enhance the effects of anti-coagulants, such as warfarin, due to the high plasma protein binding of Dexketoprofen and the inhibition of platelet function and damage to the gastroduodenal mucosa. If the combination cannot be avoided, close clinical observation and monitoring of laboratory values should be carried out.
Corticosteroids: There is an increased risk of gastrointestinal ulceration or bleeding.
Lithium (described with several NSAIDs): NSAIDs increase blood lithium levels, which may reach toxic values (decreased renal excretion of lithium). This parameter therefore requires monitoring during the initiation, adjustment and withdrawal of treatment with dexketoprofen.
Methotrexate, used at high doses of 15 mg/week or more: increased haematological toxicity of methotrexate via a decrease in its renal clearance by antiinflammatory agents in general.
Hydantoines and sulphonamides: the toxic effects of these substances may be increased.
Ketesse FC tab: Heparins: increased risk of haemorrhage (due to the inhibition of platelet function and damage to the gastroduodenal mucosa). If the combination cannot be avoided, close clinical observation and monitoring of laboratory values should be carried out.
Combinations requiring precautions: Diuretics, ACE inhibitors and angiotensin II receptor antagonists: Dexketoprofen may reduce the effect of diuretics and other antihypertensive drugs. In some patients with compromised renal function (e.g. dehydrated patients or elderly patients with compromised renal function), the co-administration of agents that inhibit cyclo-oxygenase and ACE inhibitors or angiotensin II receptor antagonists may result in further deterioration of renal function, which is usually reversible. In case of combined prescription of Dexketoprofen and a diuretic, it is essential to ensure that the patient is adequately hydrated and to monitor renal function at the start of the treatment.
Methotrexate, used at low doses, less than 15 mg/week: increased haematological toxicity of methotrexate via a decrease in its renal clearance by antiinflammatory agents in general. Weekly monitoring of blood count during the first weeks of the combination. Increased surveillance in the presence of even mildly impaired renal function, as well as in the elderly.
Pentoxyfylline: increased risk of bleeding. Intensify clinical monitoring and check bleeding time more often.
Zidovudine: risk of increased red cell line toxicity via action on reticulocytes, with severe anaemia occurring one week after the NSAID is started. Check complete blood count and reticulocyte count one to two weeks after starting treatment with the NSAID.
Sulfonylureas: NSAIDs can increase the hypoglycaemic effect of sulfonylureas by displacement from plasma protein binding sites.
Combinations needing to be taken into account: Beta-blockers: treatment with a NSAID may decrease their antihypertensive effect via inhibition of prostaglandin synthesis.
Cyclosporin and tacrolimus: nephrotoxicity may be enhanced by NSAIDs via renal prostaglandin mediated effects. During combination therapy, renal function has to be measured.
Thrombolytics: increased risk of bleeding.
Anti-platelet agents and selective serotonin reuptake inhibitors (SSRIs): increased risk of gastrointestinal bleeding.
Probenecid: plasma concentrations of dexketoprofen may be increased; this interaction can be due to an inhibitory mechanism at the site of renal tubular secretion and of glucuronoconjugation and requires adjustment of the dose of dexketoprofen.
Cardiac glycosides: NSAIDs may increase plasma glycoside concentration.
Mifepristone: Because of a theoretical risk that prostaglandin synthetase inhibitors may alter the efficacy of mifepristone, NSAIDs should not be used for 8-12 days after mifepristone administration.
Quinolone: Animal data indicate that high doses of quinolones in combination with NSAIDs can increase the risk of developing convulsions.
Anticoagulants: NSAIDs may enhance the effects of anti-coagulants, such as warfarin, due to the high plasma protein binding of Dexketoprofen and the inhibition of platelet function and damage to the gastroduodenal mucosa. If the combination cannot be avoided, close clinical observation and monitoring of laboratory values should be carried out.
Corticosteroids: There is an increased risk of gastrointestinal ulceration or bleeding.
Lithium (described with several NSAIDs): NSAIDs increase blood lithium levels, which may reach toxic values (decreased renal excretion of lithium). This parameter therefore requires monitoring during the initiation, adjustment and withdrawal of treatment with dexketoprofen.
Methotrexate, used at high doses of 15 mg/week or more: increased haematological toxicity of methotrexate via a decrease in its renal clearance by antiinflammatory agents in general.
Hydantoines and sulphonamides: the toxic effects of these substances may be increased.
Ketesse FC tab: Heparins: increased risk of haemorrhage (due to the inhibition of platelet function and damage to the gastroduodenal mucosa). If the combination cannot be avoided, close clinical observation and monitoring of laboratory values should be carried out.
Combinations requiring precautions: Diuretics, ACE inhibitors and angiotensin II receptor antagonists: Dexketoprofen may reduce the effect of diuretics and other antihypertensive drugs. In some patients with compromised renal function (e.g. dehydrated patients or elderly patients with compromised renal function), the co-administration of agents that inhibit cyclo-oxygenase and ACE inhibitors or angiotensin II receptor antagonists may result in further deterioration of renal function, which is usually reversible. In case of combined prescription of Dexketoprofen and a diuretic, it is essential to ensure that the patient is adequately hydrated and to monitor renal function at the start of the treatment.
Methotrexate, used at low doses, less than 15 mg/week: increased haematological toxicity of methotrexate via a decrease in its renal clearance by antiinflammatory agents in general. Weekly monitoring of blood count during the first weeks of the combination. Increased surveillance in the presence of even mildly impaired renal function, as well as in the elderly.
Pentoxyfylline: increased risk of bleeding. Intensify clinical monitoring and check bleeding time more often.
Zidovudine: risk of increased red cell line toxicity via action on reticulocytes, with severe anaemia occurring one week after the NSAID is started. Check complete blood count and reticulocyte count one to two weeks after starting treatment with the NSAID.
Sulfonylureas: NSAIDs can increase the hypoglycaemic effect of sulfonylureas by displacement from plasma protein binding sites.
Combinations needing to be taken into account: Beta-blockers: treatment with a NSAID may decrease their antihypertensive effect via inhibition of prostaglandin synthesis.
Cyclosporin and tacrolimus: nephrotoxicity may be enhanced by NSAIDs via renal prostaglandin mediated effects. During combination therapy, renal function has to be measured.
Thrombolytics: increased risk of bleeding.
Anti-platelet agents and selective serotonin reuptake inhibitors (SSRIs): increased risk of gastrointestinal bleeding.
Probenecid: plasma concentrations of dexketoprofen may be increased; this interaction can be due to an inhibitory mechanism at the site of renal tubular secretion and of glucuronoconjugation and requires adjustment of the dose of dexketoprofen.
Cardiac glycosides: NSAIDs may increase plasma glycoside concentration.
Mifepristone: Because of a theoretical risk that prostaglandin synthetase inhibitors may alter the efficacy of mifepristone, NSAIDs should not be used for 8-12 days after mifepristone administration.
Quinolone: Animal data indicate that high doses of quinolones in combination with NSAIDs can increase the risk of developing convulsions.
Caution For Usage
Instructions for Use and Handling: Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion has shown to be compatible when mixed in small volumes (e.g. in a syringe) with injectable solutions of heparin, lidocaine, morphine and theophylline.
For administration as intravenous infusion the content of one ampoule (2 mL) of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should be diluted in a volume of 30 to 100 mL of normal saline, glucose or Ringer lactate solution. The solution should be diluted aseptically and protected from natural daylight. The diluted solution is a clear solution.
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion, diluted in a volume of 100 mL of normal saline or glucose solution has shown to be compatible with the following medicinal products: dopamine, heparin, hydroxyzine, lidocaine, morphine, pethidine and theophylline.
No sorption of the active ingredient has been found when diluted solutions of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion have been stored in plastic bags or administration devices made of Ethyl Vinyl Acetate (EVA), Cellulose Propionate (CP), Low Density PolyEthylene (LDPE) and PolyVinyl Chloride (PVC).
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion is for single use only and any unused solution should be discarded. Prior to administration, the solution should be inspected visually to make sure it is clear and colourless: it should not be used if particulate matter is observed.
Incompatibilities: Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion must not be mixed in a small volume (e.g. in a syringe) with solutions of dopamine, promethazine, pentazocine, pethidine or hydroxyzine, as this will result in a precipitation of the solution. The diluted solutions for infusion obtained as stated in "Instructions for use and handling" must not be mixed with promethazine or pentazocine.
This medicinal product must not be mixed with other medicinal products except those mentioned in Instructions for use and handling.
For administration as intravenous infusion the content of one ampoule (2 mL) of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion should be diluted in a volume of 30 to 100 mL of normal saline, glucose or Ringer lactate solution. The solution should be diluted aseptically and protected from natural daylight. The diluted solution is a clear solution.
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion, diluted in a volume of 100 mL of normal saline or glucose solution has shown to be compatible with the following medicinal products: dopamine, heparin, hydroxyzine, lidocaine, morphine, pethidine and theophylline.
No sorption of the active ingredient has been found when diluted solutions of Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion have been stored in plastic bags or administration devices made of Ethyl Vinyl Acetate (EVA), Cellulose Propionate (CP), Low Density PolyEthylene (LDPE) and PolyVinyl Chloride (PVC).
Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion is for single use only and any unused solution should be discarded. Prior to administration, the solution should be inspected visually to make sure it is clear and colourless: it should not be used if particulate matter is observed.
Incompatibilities: Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion must not be mixed in a small volume (e.g. in a syringe) with solutions of dopamine, promethazine, pentazocine, pethidine or hydroxyzine, as this will result in a precipitation of the solution. The diluted solutions for infusion obtained as stated in "Instructions for use and handling" must not be mixed with promethazine or pentazocine.
This medicinal product must not be mixed with other medicinal products except those mentioned in Instructions for use and handling.
Storage
Store at temperatures not exceeding 30°C. Protect from light.
Shelf-Life: Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): After dilution according to directions given in below (see Instructions for use and handling under Cautions for Usage), the diluted solution, provided it is adequately protected from natural daylight, has been shown to be chemically stable for 24 hours, when stored at 25°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless dilution has taken place in controlled and validated aseptic conditions.
Shelf-Life: Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): After dilution according to directions given in below (see Instructions for use and handling under Cautions for Usage), the diluted solution, provided it is adequately protected from natural daylight, has been shown to be chemically stable for 24 hours, when stored at 25°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless dilution has taken place in controlled and validated aseptic conditions.
Action
Pharmacology: Pharmacodynamics: Dexketoprofen trometamol is the tromethamine salt of S-(+)-2-(3-benzoylphenyl) propionic acid, an analgesic, anti-inflammatory and antipyretic drug, which belongs to the non-steroidal anti-inflammatory group of drugs (M01AE).
The mechanism of action of non-steroidal anti-inflammatory drugs is related to the reduction of prostaglandin synthesis by the inhibition of cyclooxygenase pathway. Specifically, there is an inhibition of the transformation of arachidonic acid into cyclic endoperoxides, PGG2 and PGH2, which produce prostaglandins PGE1, PGE2, PGF2α and PGD2 and also prostacyclin PGI2 and thromboxanes (TxA2 and TxB2). Furthermore, the inhibition of the synthesis of prostaglandins could affect other inflammation mediators such as kinins, causing an indirect action which would be additional to the direct action.
Dexketoprofen has been demonstrated to be an inhibitor for COX-1 and COX-2 activities in experimental animals and humans.
Clinical studies performed on several pain models demonstrated effective analgesic activity of Dexketoprofen trometamol.
Ketesse FC tab: The onset of the analgesic activity was obtained in some studies at 30 minutes post-administration. The analgesic effect persists for 4 to 6 hours.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): The analgesic efficacy of intramuscular and intravenous dexketoprofen trometamol in the management of moderate to severe pain was investigated in several surgical pain models (orthopaedic and gynaecologic/abdominal surgery) as well as in musculo-skeletal pain (acute low back pain model) and renal colic.
In the studies performed, the onset of analgesic effect was rapid, and within the first 45 minutes the peak analgesic effect occurred. Duration of analgesic effect after the administration of 50 mg of dexketoprofen is usually 8 hours.
Clinical studies in postoperative pain management have demonstrated that Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion when used in combination with opioids significantly reduced opioid consumption. In the post-operative pain studies where patients received morphine by a patient controlled analgesia device, patients treated with dexketoprofen required significantly less morphine (between 30-45% less) than patients in the placebo group.
Pharmacokinetics Ketesse FC tab: After oral administration of Dexketoprofen trometamol to humans, the Cmax is reached at 30 min (range 15 to 60 min).
The distribution half-life and elimination half-life values of Dexketoprofen trometamol are 0.35 and 1.65 hours, respectively. As with other drugs with a high plasma protein binding (99%), its volume of distribution has a mean value below 0.25 L/kg. The main elimination route for Dexketoprofen is glucuronide conjugation followed by renal excretion.
After administration of Dexketoprofen trometamol only the S-(+) enantiomer is obtained in urine, demonstrating that no conversion to the R-(-) enantiomer occurs in humans.
In multiple-dose pharmacokinetic studies, it was observed that the AUC after the last administration is not different from that obtained following a single dose, indicating that no drug accumulation occurs.
When administered concomitantly with food, the AUC does not change, however the Cmax of Dexketoprofen trometamol decreases and its absorption rate is delayed (increased tmax).
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): After intramuscular administration of dexketoprofen trometamol to humans, the peak concentrations are reached at 20 minutes (range 10 to 45 min). For 25 to 50 mg single doses the area under the curve has been shown to be dose proportional after both intramuscular and intravenous administration.
In multiple-dose pharmacokinetic studies, it was observed that Cmax and AUC after the last intramuscular or intravenous administration were not different from that obtained following a single dose, indicating that no drug accumulation occurs.
As with other medicinal products with a high plasma protein binding (99%), the volume of distribution has a mean value below 0.25 L/kg. The distribution half-life was approximately 0.35 hours and the elimination half-life ranged between 1-2.7 hours. The main elimination route for dexketoprofen is glucuronide conjugation followed by renal excretion.
After administration of dexketoprofen trometamol only the S-(+) enantiomer is obtained in urine, demonstrating that no conversion to the R-(-) enantiomer occurs in humans.
In healthy elderly subjects (65 years and older), exposure was significantly higher than in young volunteers after single and repeated oral doses (up to 55%) whereas there was no statistically significant difference in peak concentrations and time to reach peak concentration. The mean elimination half-life was prolonged after single and repeated doses (up to 48%), and the apparent total clearance was reduced.
Toxicology: Preclinical Safety Data: Ketesse FC tab:Pre-clinical data revealed no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction and immunopharmacology. The chronic toxicity studies carried out in mice and monkeys gave a No Observed Adverse Effect Level (NOAEL) of 3 mg/kg/day. The main adverse effect observed at high doses was gastrointestinal erosions and ulcers that developed dose-dependently.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Preclinical data based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction and immunopharmacology revealed no special hazard for humans in addition to those above mentioned. The chronic toxicity studies carried out in mice and monkeys gave a No Observed Adverse Effect Level (NOAEL) of 3 mg/kg/day. The main adverse effect observed at high doses was gastrointestinal erosions and ulcers in a dose related manner.
As it has been recognised for the whole pharmacological class of NSAIDs, dexketoprofen trometamol may cause changes of embryo-foetal survival in animal models, both indirectly, through the gastrointestinal toxicity on the pregnant mothers, and directly upon the development of the foetus.
The mechanism of action of non-steroidal anti-inflammatory drugs is related to the reduction of prostaglandin synthesis by the inhibition of cyclooxygenase pathway. Specifically, there is an inhibition of the transformation of arachidonic acid into cyclic endoperoxides, PGG2 and PGH2, which produce prostaglandins PGE1, PGE2, PGF2α and PGD2 and also prostacyclin PGI2 and thromboxanes (TxA2 and TxB2). Furthermore, the inhibition of the synthesis of prostaglandins could affect other inflammation mediators such as kinins, causing an indirect action which would be additional to the direct action.
Dexketoprofen has been demonstrated to be an inhibitor for COX-1 and COX-2 activities in experimental animals and humans.
Clinical studies performed on several pain models demonstrated effective analgesic activity of Dexketoprofen trometamol.
Ketesse FC tab: The onset of the analgesic activity was obtained in some studies at 30 minutes post-administration. The analgesic effect persists for 4 to 6 hours.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): The analgesic efficacy of intramuscular and intravenous dexketoprofen trometamol in the management of moderate to severe pain was investigated in several surgical pain models (orthopaedic and gynaecologic/abdominal surgery) as well as in musculo-skeletal pain (acute low back pain model) and renal colic.
In the studies performed, the onset of analgesic effect was rapid, and within the first 45 minutes the peak analgesic effect occurred. Duration of analgesic effect after the administration of 50 mg of dexketoprofen is usually 8 hours.
Clinical studies in postoperative pain management have demonstrated that Dexketoprofen (KETESSE) Solution for Injection or concentrate for solution for infusion when used in combination with opioids significantly reduced opioid consumption. In the post-operative pain studies where patients received morphine by a patient controlled analgesia device, patients treated with dexketoprofen required significantly less morphine (between 30-45% less) than patients in the placebo group.
Pharmacokinetics Ketesse FC tab: After oral administration of Dexketoprofen trometamol to humans, the Cmax is reached at 30 min (range 15 to 60 min).
The distribution half-life and elimination half-life values of Dexketoprofen trometamol are 0.35 and 1.65 hours, respectively. As with other drugs with a high plasma protein binding (99%), its volume of distribution has a mean value below 0.25 L/kg. The main elimination route for Dexketoprofen is glucuronide conjugation followed by renal excretion.
After administration of Dexketoprofen trometamol only the S-(+) enantiomer is obtained in urine, demonstrating that no conversion to the R-(-) enantiomer occurs in humans.
In multiple-dose pharmacokinetic studies, it was observed that the AUC after the last administration is not different from that obtained following a single dose, indicating that no drug accumulation occurs.
When administered concomitantly with food, the AUC does not change, however the Cmax of Dexketoprofen trometamol decreases and its absorption rate is delayed (increased tmax).
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): After intramuscular administration of dexketoprofen trometamol to humans, the peak concentrations are reached at 20 minutes (range 10 to 45 min). For 25 to 50 mg single doses the area under the curve has been shown to be dose proportional after both intramuscular and intravenous administration.
In multiple-dose pharmacokinetic studies, it was observed that Cmax and AUC after the last intramuscular or intravenous administration were not different from that obtained following a single dose, indicating that no drug accumulation occurs.
As with other medicinal products with a high plasma protein binding (99%), the volume of distribution has a mean value below 0.25 L/kg. The distribution half-life was approximately 0.35 hours and the elimination half-life ranged between 1-2.7 hours. The main elimination route for dexketoprofen is glucuronide conjugation followed by renal excretion.
After administration of dexketoprofen trometamol only the S-(+) enantiomer is obtained in urine, demonstrating that no conversion to the R-(-) enantiomer occurs in humans.
In healthy elderly subjects (65 years and older), exposure was significantly higher than in young volunteers after single and repeated oral doses (up to 55%) whereas there was no statistically significant difference in peak concentrations and time to reach peak concentration. The mean elimination half-life was prolonged after single and repeated doses (up to 48%), and the apparent total clearance was reduced.
Toxicology: Preclinical Safety Data: Ketesse FC tab:Pre-clinical data revealed no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction and immunopharmacology. The chronic toxicity studies carried out in mice and monkeys gave a No Observed Adverse Effect Level (NOAEL) of 3 mg/kg/day. The main adverse effect observed at high doses was gastrointestinal erosions and ulcers that developed dose-dependently.
Ketesse Solution for Injection or Concentrate for Solution for Infusion (I.V./I.M.): Preclinical data based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction and immunopharmacology revealed no special hazard for humans in addition to those above mentioned. The chronic toxicity studies carried out in mice and monkeys gave a No Observed Adverse Effect Level (NOAEL) of 3 mg/kg/day. The main adverse effect observed at high doses was gastrointestinal erosions and ulcers in a dose related manner.
As it has been recognised for the whole pharmacological class of NSAIDs, dexketoprofen trometamol may cause changes of embryo-foetal survival in animal models, both indirectly, through the gastrointestinal toxicity on the pregnant mothers, and directly upon the development of the foetus.
MedsGo Class
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Features
Dosage
25 mg
Ingredients
- Dexketoprofen
Packaging
Film-Coated Tablet 1's
Generic Name
Dexketoprofen trometamol
Registration Number
DR-XY36275
Classification
Prescription Drug (RX)
Reviews
No reviews found
Pharmacist answers to questions about KETESSE Dexketoprofen Trometamol 25mg Film-Coated Tablet 1's
Questions



