Indications/Uses
Treatment of: Onychomycosis (fungal infection of the nail) caused by dermatophyte fungi.
Tinea capitis.
Fungal infections of the skin (Tinea corporis, Tinea cruris, Tinea pedis) and yeast infections of the skin caused by the genus Candida (e.g. Candida albicans) where oral therapy is generally considered appropriate owing to the site, severity or extent of the infection.
Note: In contrast to the topical preparation, oral terbinafine is not effective in Pityriasis versicolor (also known as Tinea versicolor).
Tinea capitis.
Fungal infections of the skin (Tinea corporis, Tinea cruris, Tinea pedis) and yeast infections of the skin caused by the genus Candida (e.g. Candida albicans) where oral therapy is generally considered appropriate owing to the site, severity or extent of the infection.
Note: In contrast to the topical preparation, oral terbinafine is not effective in Pityriasis versicolor (also known as Tinea versicolor).
Dosage/Direction for Use
Dosage: The duration of treatment varies according to the indication and the severity of the infection.
Adults: 250 mg once daily.
Skin infections: Recommended duration of treatment: Tinea pedis (interdigital, plantar/moccasin type): 2 to 6 weeks.
Tinea corporis, T. cruris: 2 to 4 weeks.
Cutaneous candidiasis: 2 to 4 weeks.
Complete resolution of the signs and symptoms of infection may not occur until several weeks after mycological cure.
Hair and scalp infections: Recommended duration of treatment: Tinea capitis: 4 weeks.
Tinea capitis occurs primarily in children.
Onychomycosis: For most patients the duration of successful treatment is 6 to 12 weeks.
Fingernail onychomycosis: Six weeks of therapy is sufficient for fingernail infections in most cases.
Toenail onychomycosis: Twelve weeks of therapy is sufficient for toenail infections in most cases.
Some patients with poor nail outgrowth may require longer treatment. The optimal clinical effect is seen some months after mycological cure and cessation of treatment. This is related to the period required for outgrowth of healthy nail.
Special populations: Renal impairment: The use of terbinafine (Lamisil) tablets has not been adequately studied in patients with renal impairment and is therefore not recommended in this population (see PRECAUTIONS and Pharmacology: PHARMACOKINETICS under Actions).
Hepatic impairment: Terbinafine (Lamisil) tablets are contraindicated for patients with chronic or active liver disease (see PRECAUTIONS).
Pediatric patients: No data are available in children under two years of age (usually <12 kg).
Children weighing <20 kg 62.5 mg (half a 125 mg tablet) once daily.
Children weighing 20 to 40 kg 125 mg (one 125 mg tablet) once daily.
Children weighing >40 kg 250 mg (two 125 mg tablets) once daily.
Geriatric patients: There is no evidence to suggest that elderly patients (aged 65 years and above) require different dosages or experience different side effects than younger patients. When prescribing terbinafine (Lamisil) tablets for patients in this age group, the possibility of pre-existing impairment of liver or kidney function should be considered (see PRECAUTIONS).
Method of administration: The scored tablets are taken orally with water. They should preferably be taken at the same time each day and can be taken on an empty stomach or after a meal.
Adults: 250 mg once daily.
Skin infections: Recommended duration of treatment: Tinea pedis (interdigital, plantar/moccasin type): 2 to 6 weeks.
Tinea corporis, T. cruris: 2 to 4 weeks.
Cutaneous candidiasis: 2 to 4 weeks.
Complete resolution of the signs and symptoms of infection may not occur until several weeks after mycological cure.
Hair and scalp infections: Recommended duration of treatment: Tinea capitis: 4 weeks.
Tinea capitis occurs primarily in children.
Onychomycosis: For most patients the duration of successful treatment is 6 to 12 weeks.
Fingernail onychomycosis: Six weeks of therapy is sufficient for fingernail infections in most cases.
Toenail onychomycosis: Twelve weeks of therapy is sufficient for toenail infections in most cases.
Some patients with poor nail outgrowth may require longer treatment. The optimal clinical effect is seen some months after mycological cure and cessation of treatment. This is related to the period required for outgrowth of healthy nail.
Special populations: Renal impairment: The use of terbinafine (Lamisil) tablets has not been adequately studied in patients with renal impairment and is therefore not recommended in this population (see PRECAUTIONS and Pharmacology: PHARMACOKINETICS under Actions).
Hepatic impairment: Terbinafine (Lamisil) tablets are contraindicated for patients with chronic or active liver disease (see PRECAUTIONS).
Pediatric patients: No data are available in children under two years of age (usually <12 kg).
Children weighing <20 kg 62.5 mg (half a 125 mg tablet) once daily.
Children weighing 20 to 40 kg 125 mg (one 125 mg tablet) once daily.
Children weighing >40 kg 250 mg (two 125 mg tablets) once daily.
Geriatric patients: There is no evidence to suggest that elderly patients (aged 65 years and above) require different dosages or experience different side effects than younger patients. When prescribing terbinafine (Lamisil) tablets for patients in this age group, the possibility of pre-existing impairment of liver or kidney function should be considered (see PRECAUTIONS).
Method of administration: The scored tablets are taken orally with water. They should preferably be taken at the same time each day and can be taken on an empty stomach or after a meal.
Overdosage
A few cases of overdosage (up to 5 g) have been reported, giving rise to headache, nausea, epigastric pain and dizziness.
The recommended treatment of overdosage consists of eliminating the drug, primarily by the administration of activated charcoal, and giving symptomatic supportive therapy, if needed.
The recommended treatment of overdosage consists of eliminating the drug, primarily by the administration of activated charcoal, and giving symptomatic supportive therapy, if needed.
Administration
May be taken with or without food: Take on an empty stomach or after a meal. Take at the same time each day.
Contraindications
Known hypersensitivity to terbinafine or to any of the excipients.
Chronic or active hepatic disease.
Chronic or active hepatic disease.
Special Precautions
Liver function: Terbinafine (Lamisil) tablets are contraindicated for patients with chronic or active hepatic disease. Before prescribing terbinafine (Lamisil) tablets, liver function tests should be performed since hepatotoxicity may occur in patients with and without pre-existing liver disease. Therefore periodic monitoring (after 4-6 weeks of treatment) of liver function tests is recommended. Drug should be immediately discontinued in case of elevation of liver function tests. Very rare cases of serious liver failure (some with a fatal outcome, or requiring liver transplant) have been reported in patients treated with terbinafine (Lamisil) tablets. In the majority of hepatic failure cases the patients had serious underlying systemic conditions (see CONTRAINDICATIONS and ADVERSE REACTIONS). Patients prescribed terbinafine (Lamisil) tablets should be warned to report immediately any symptoms of unexplained persistent nausea, decreased appetite, fatigue, vomiting, right upper abdominal pain, or jaundice, dark urine or pale feces. Patients with these symptoms should discontinue taking oral terbinafine and the patient's hepatic function should be immediately evaluated.
Dermatological effects: Serious skin reactions (e.g. Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms) have been very rarely reported. If progressive skin rash occurs, treatment should be discontinued.
Terbinafine should be used with caution in patients with pre-existing psoriasis or lupus erythematosus as precipitation and exacerbation of psoriasis and cutaneous and systemic lupus erythematosus have been reported in a post-marketing setting.
Haematological effects: Very rare cases of blood dyscrasias (neutropenia, agranulocytosis, thrombocytopenia, pancytopenia) have been reported in patients treated with terbinafine (Lamisil) tablets. Etiology of any blood dyscrasias that occur in patients treated with terbinafine (Lamisil) tablets should be evaluated and consideration should be given for a possible change in medication regimen, including discontinuation of treatment.
Renal function: In patients with renal impairment (creatinine clearance less than 50 mL/min or serum creatinine of more than 300 micro mol/L) the use of terbinafine (Lamisil) tablets has not been adequately studied, and therefore, is not recommended (see Pharmacology: PHARMACOKINETICS under Actions).
Interactions: In vitro and in vivo studies have shown that terbinafine inhibits the CYP2D6 metabolism. Therefore, patients receiving concomitant treatment with drugs predominantly metabolized by CYP2D6, e.g. certain members of the following drug classes, tricyclic antidepressants (TCAs), beta-blockers, selective serotonine reuptake inhibitors (SSRIs), antiarrhythmics (including class 1A, 1B and 1C) and monoamine oxidase inhibitors (MAO-Is) Type B, should be followed up, especially if the co-administered drug has a narrow therapeutic window (see INTERACTIONS).
Dermatological effects: Serious skin reactions (e.g. Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms) have been very rarely reported. If progressive skin rash occurs, treatment should be discontinued.
Terbinafine should be used with caution in patients with pre-existing psoriasis or lupus erythematosus as precipitation and exacerbation of psoriasis and cutaneous and systemic lupus erythematosus have been reported in a post-marketing setting.
Haematological effects: Very rare cases of blood dyscrasias (neutropenia, agranulocytosis, thrombocytopenia, pancytopenia) have been reported in patients treated with terbinafine (Lamisil) tablets. Etiology of any blood dyscrasias that occur in patients treated with terbinafine (Lamisil) tablets should be evaluated and consideration should be given for a possible change in medication regimen, including discontinuation of treatment.
Renal function: In patients with renal impairment (creatinine clearance less than 50 mL/min or serum creatinine of more than 300 micro mol/L) the use of terbinafine (Lamisil) tablets has not been adequately studied, and therefore, is not recommended (see Pharmacology: PHARMACOKINETICS under Actions).
Interactions: In vitro and in vivo studies have shown that terbinafine inhibits the CYP2D6 metabolism. Therefore, patients receiving concomitant treatment with drugs predominantly metabolized by CYP2D6, e.g. certain members of the following drug classes, tricyclic antidepressants (TCAs), beta-blockers, selective serotonine reuptake inhibitors (SSRIs), antiarrhythmics (including class 1A, 1B and 1C) and monoamine oxidase inhibitors (MAO-Is) Type B, should be followed up, especially if the co-administered drug has a narrow therapeutic window (see INTERACTIONS).
Use In Pregnancy & Lactation
Women of child-bearing potential: Some cases of menstrual irregularities have been reported in patients taking terbinafine (Lamisil) tablets concomitantly with oral contraceptives, although the incidence of these disorders remains within the background incidence of patients taking oral contraceptives alone.
There are no data to support special recommendations for women of child-bearing potential.
Pregnancy: Fetal toxicity studies with terbinafine in animals suggest no adverse effects. Since documented clinical experience in pregnant women is very limited, terbinafine (Lamisil) tablets should not be used during pregnancy unless the potential benefits outweigh any potential risks.
Breast-feeding: Terbinafine is excreted in breast milk; mothers receiving oral treatment with terbinafine (Lamisil) should therefore not breast-feed.
Fertility: There is no relevant information from human experience. Fertility studies in rats indicated no adverse findings in fertility or reproductive performance.
There are no data to support special recommendations for women of child-bearing potential.
Pregnancy: Fetal toxicity studies with terbinafine in animals suggest no adverse effects. Since documented clinical experience in pregnant women is very limited, terbinafine (Lamisil) tablets should not be used during pregnancy unless the potential benefits outweigh any potential risks.
Breast-feeding: Terbinafine is excreted in breast milk; mothers receiving oral treatment with terbinafine (Lamisil) should therefore not breast-feed.
Fertility: There is no relevant information from human experience. Fertility studies in rats indicated no adverse findings in fertility or reproductive performance.
Adverse Reactions
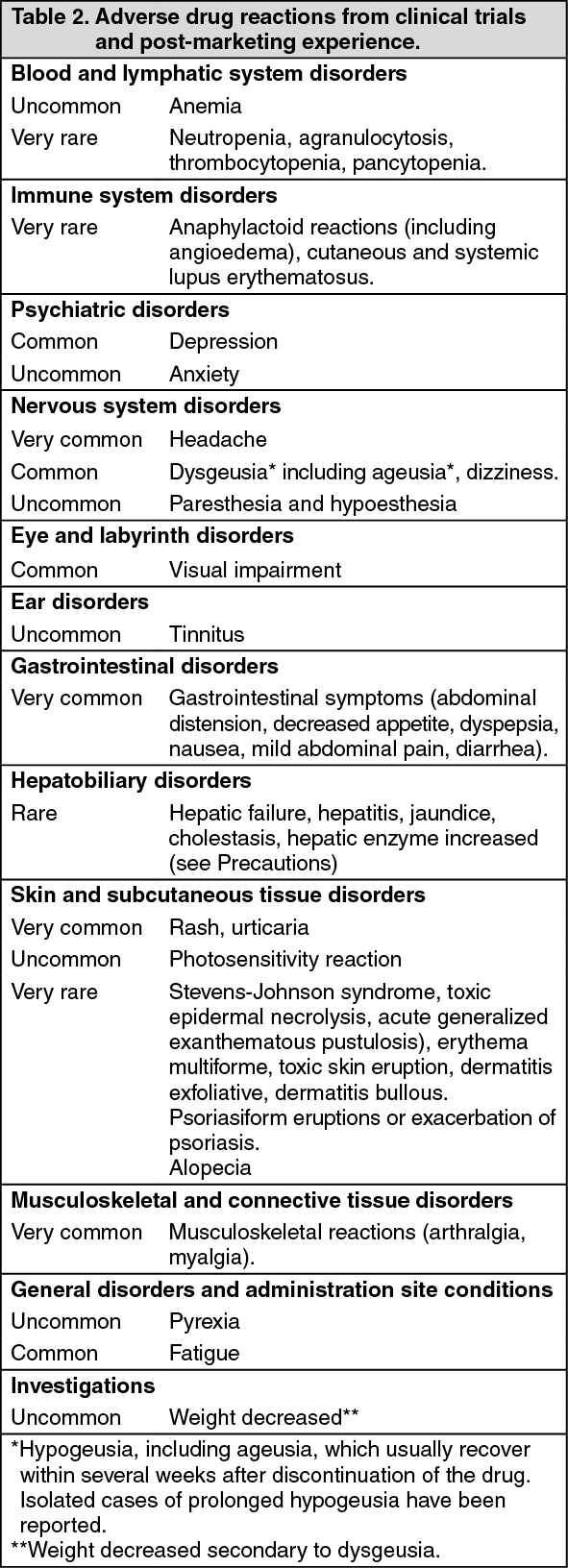
Adverse drug reactions from clinical trials or post-marketing experience (Table 2) are listed by MedDRA system organ class. Within each system organ class, the adverse drug reactions are ranked by frequency, with the most frequent reactions first. Within each frequency grouping, adverse drug reactions are presented in order of decreasing seriousness. In addition, the corresponding frequency category for each adverse drug reaction is based on the following convention (CIOMS III): very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000). (See Table 2.)
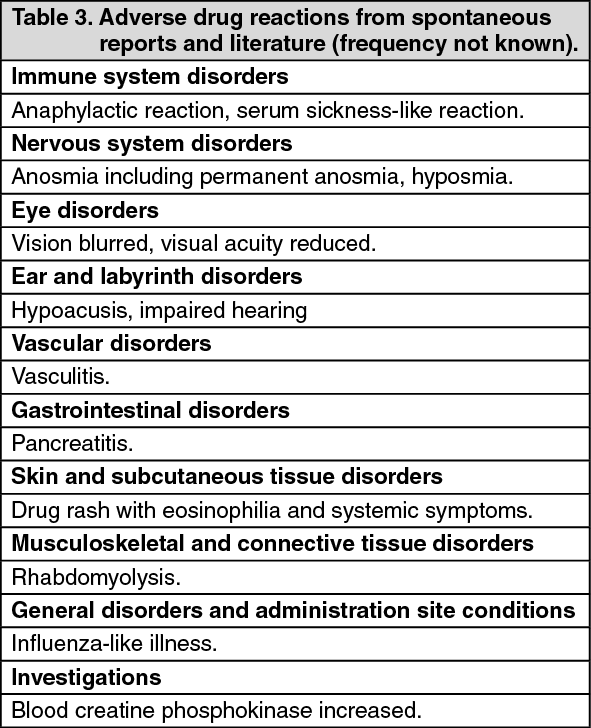
Adverse drug reactions from spontaneous reports and literature cases (frequency not known): The following adverse drug reactions have been derived from post-marketing experience with terbinafine (Lamisil) via spontaneous case reports and literature cases. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency which is therefore categorized as not known. Adverse drug reactions are listed according to system organ classes in MedDRA. Within each system organ class, ADRs are presented in order of decreasing seriousness. (See Table 3.)
Drug Interactions
Observed interactions to be considered: Interactions affecting the use of terbinafine (Lamisil): The plasma clearance of terbinafine may be accelerated by drugs, which induce metabolism and may be inhibited by drugs, which inhibit cytochrome P450. Where co-administration of such agents is necessary, the dosage may need to be adjusted accordingly.
The following medicinal products may increase the effect or plasma concentration of terbinafine: Cimetidine decreased the clearance of terbinafine by 33%.
Fluconazole increased the Cmax and AUC of terbinafine by 52% and 69% respectively, due to inhibition of both CYP2C9 and CYP3A4 enzymes. Similar increase in exposure may occur when other drugs which inhibit both CYP2C9 and CYP3A4 such as ketoconazole and amiodarone are concomitantly administered with terbinafine.
The following medicinal products may decrease the effect or plasma concentration of terbinafine: Rifampicin increased the clearance of terbinafine by 100%.
Interactions resulting in effects on other medicinal products: Terbinafine may increase the effect or plasma concentration of the following medicinal products: Compounds predominantly metabolized by CYP2D6: In vitro and in vivo studies have shown that terbinafine inhibits the CYP2D6-mediated metabolism. This finding may be of clinical relevance for compounds predominantly metabolized by CYP2D6, e.g. certain members of the following drug classes, tricyclic antidepressants (TCAs), beta-blockers, selective serotonine reuptake inhibitors (SSRIs), antiarrhythmics (including class 1A, 1B and 1C) and monoamine oxidase inhibitors (MAO-Is) Type B, especially if they also have a narrow therapeutic window (see PRECAUTIONS).
Terbinafine decreased the clearance of desipramine by 82% (see PRECAUTIONS).
In studies in healthy subjects characterized as extensive metabolizers of dextromethorphan (antitussive drug and CYP2D6 probe substrate), terbinafine increased the dextromethorphan/dextrorphan metabolic ratio in urine by 16- to 97-fold on average. Thus, terbinafine may convert extensive CYP2D6 metabolizers (genotype) to poor metabolizer phenotype status.
Caffeine: Terbinafine decreased the clearance of caffeine administered intravenously by 19%.
Information on other drugs concomitantly used with terbinafine resulting in no or negligible interactions: According to the results from studies undertaken in vitro and in healthy volunteers, terbinafine shows negligible potential for inhibiting or enhancing the clearance of most drugs that are metabolized via the cytochrome P450 system (e.g. terfenadine, triazolam, tolbutamide or oral contraceptives) with exception of those metabolized through CYP2D6 (see as follows).
Terbinafine does not interfere with the clearance of antipyrine or digoxin.
There was no effect of terbinafine on the pharmacokinetics of fluconazole. Further there was no clinically relevant interaction between terbinafine and the potential co-medications cotrimoxazole (trimethoprim and sulfamethoxazole), zidovudine or theophylline.
Some cases of menstrual irregularities have been reported in patients taking terbinafine (Lamisil) tablets concomitantly with oral contraceptives, although the incidence of these disorders remains within the background incidence of patients taking oral contraceptives alone.
Terbinafine may decrease the effect or plasma concentration of the following medicinal products: Terbinafine increased the clearance of ciclosporin by 15%.
Drug-food/drink interactions: The bioavailability of terbinafine is moderately affected by food (increase in the AUC of less than 20%), but not sufficiently to require dose adjustments.
The following medicinal products may increase the effect or plasma concentration of terbinafine: Cimetidine decreased the clearance of terbinafine by 33%.
Fluconazole increased the Cmax and AUC of terbinafine by 52% and 69% respectively, due to inhibition of both CYP2C9 and CYP3A4 enzymes. Similar increase in exposure may occur when other drugs which inhibit both CYP2C9 and CYP3A4 such as ketoconazole and amiodarone are concomitantly administered with terbinafine.
The following medicinal products may decrease the effect or plasma concentration of terbinafine: Rifampicin increased the clearance of terbinafine by 100%.
Interactions resulting in effects on other medicinal products: Terbinafine may increase the effect or plasma concentration of the following medicinal products: Compounds predominantly metabolized by CYP2D6: In vitro and in vivo studies have shown that terbinafine inhibits the CYP2D6-mediated metabolism. This finding may be of clinical relevance for compounds predominantly metabolized by CYP2D6, e.g. certain members of the following drug classes, tricyclic antidepressants (TCAs), beta-blockers, selective serotonine reuptake inhibitors (SSRIs), antiarrhythmics (including class 1A, 1B and 1C) and monoamine oxidase inhibitors (MAO-Is) Type B, especially if they also have a narrow therapeutic window (see PRECAUTIONS).
Terbinafine decreased the clearance of desipramine by 82% (see PRECAUTIONS).
In studies in healthy subjects characterized as extensive metabolizers of dextromethorphan (antitussive drug and CYP2D6 probe substrate), terbinafine increased the dextromethorphan/dextrorphan metabolic ratio in urine by 16- to 97-fold on average. Thus, terbinafine may convert extensive CYP2D6 metabolizers (genotype) to poor metabolizer phenotype status.
Caffeine: Terbinafine decreased the clearance of caffeine administered intravenously by 19%.
Information on other drugs concomitantly used with terbinafine resulting in no or negligible interactions: According to the results from studies undertaken in vitro and in healthy volunteers, terbinafine shows negligible potential for inhibiting or enhancing the clearance of most drugs that are metabolized via the cytochrome P450 system (e.g. terfenadine, triazolam, tolbutamide or oral contraceptives) with exception of those metabolized through CYP2D6 (see as follows).
Terbinafine does not interfere with the clearance of antipyrine or digoxin.
There was no effect of terbinafine on the pharmacokinetics of fluconazole. Further there was no clinically relevant interaction between terbinafine and the potential co-medications cotrimoxazole (trimethoprim and sulfamethoxazole), zidovudine or theophylline.
Some cases of menstrual irregularities have been reported in patients taking terbinafine (Lamisil) tablets concomitantly with oral contraceptives, although the incidence of these disorders remains within the background incidence of patients taking oral contraceptives alone.
Terbinafine may decrease the effect or plasma concentration of the following medicinal products: Terbinafine increased the clearance of ciclosporin by 15%.
Drug-food/drink interactions: The bioavailability of terbinafine is moderately affected by food (increase in the AUC of less than 20%), but not sufficiently to require dose adjustments.
Caution For Usage
Incompatibilities: None known.
Storage
Store at temperatures not exceeding 30°C.
Action
Pharmacology: Mechanism of action: Terbinafine is an allylamine which has a broad spectrum of activity against fungal pathogens of the skin, hair and nails including dermatophytes such as Trichophyton (e.g. T. rubrum, T. mentagrophytes, T. verrucosum, T. tonsurans, T. violaceum), Microsporum (e.g. M. canis), Epidermophyton floccosum, and yeasts of the genera Candida (e.g. C. albicans) and Malassezia. At low concentrations terbinafine is fungicidal against dermatophytes, moulds and certain dimorphic fungi. Its activity against yeasts is fungicidal or fungistatic, depending on the species.
Terbinafine interferes specifically with fungal sterol biosynthesis at an early step. This leads to a deficiency in ergosterol and to an intracellular accumulation of squalene, resulting in fungal cell death. Terbinafine acts by inhibition of squalene epoxidase in the fungal cell membrane. The enzyme squalene epoxidase is not linked to the cytochrome P450 system.
Pharmacodynamics: When given orally, terbinafine accumulates in skin, hair and nails at levels associated with fungicidal activity.
Clinical Studies: Onychomycosis: The efficacy of terbinafine (Lamisil) tablets in the treatment of onychomycosis is illustrated by the response of patients with toenail and/or fingernail infections who participated in three US/Canadian placebo-controlled clinical trials (SFD301, SF5 and SF1508).
Results of the first toenail study, as assessed at week 48 (12 weeks of treatment with 36 weeks follow-up after completion of therapy), demonstrated mycological cure, defined as simultaneous occurrence of negative KOH plus negative culture, in 70% of patients. Fifty-nine percent (59%) of patients experienced effective treatment (mycological cure plus 0% nail involvement or >5mm of new unaffected nail growth); 38% of patients demonstrated mycological cure plus clinical cure (0% nail involvement).
In a second toenail study of dermatophytic onychomycosis, in which non-dermatophytes were also cultured, similar efficacy against the dermatophytes was demonstrated. The pathogenic role of the non-dermatophytes cultured in the presence of dermatophytic onychomycosis has not been established. The clinical significance of this association is unknown.
Results of the fingernail study, as assessed at week 24 (6 weeks of treatment with 18 weeks follow-up after completion of therapy), demonstrated mycological cure in 79% of patients, effective treatment in 75% of the patients, and mycological cure plus clinical cure in 59% of the patients.
The mean time to treatment success for onychomycosis was approximately 10 months for the first toenail study and 4 months for the fingernail study. In the first toenail study, for patients evaluated at least six months after achieving clinical cure and at least one year after completing terbinafine (Lamisil) therapy, the clinical relapse rate was approximately 15%.
Tinea capitis: In the three comparative efficacy studies SF 8001, SFE 304, SF 8002 oral terbinafine (Lamisil) (62.5 - 250 mg daily) was given to a total of 117 evaluable patients, of whom over 97% were children. Single daily doses were given after the evening meal for 4 weeks (Terbinafine (Lamisil)) or 8 weeks (griseofulvin). Efficacy, demonstrated by negative mycology tests and a reduction in symptomatology, was evaluated at 8 weeks and at the follow-up examination (Week 12 for Studies SF 8001 and SFE 304, Week 24 for Study SF 8002). Negative mycology test results at follow-up were achieved by 85%, 88% and 72% of patients given terbinafine (Lamisil) in the three studies - the corresponding figures for griseofulvin were 73%, 89% and 69%. The derived variable "effective treatment" (negative mycology plus no, or only mild, symptoms and signs) was achieved in 82%, 78% and 69% of terbinafine-treated patients, compared with 66%, 74% and 59% in patients given griseofulvin; the difference was statistically significant in favor of terbinafine in Study SF 8001.
Two phase II treatment duration finding studies totaling 342 patients (mostly children) with T. capitis have been completed.
A 12-week randomized, double-blind, parallel group study was conducted in the United States and in Canada in children with Tinea capitis infection due to Trichophyton species (SFO327C T201). The objective of the study was to determine the optimal duration (1, 2 or 4 weeks) and safety of treatment with terbinafine (Lamisil) (tablets), given at weight adjusted doses once daily.
A second 16-week randomized, active-controlled, parallel-group, multicenter study was conducted in Europe in patients with Tinea capitis (>4 years) due to Microsporum species. The terbinafine (Lamisil) treatment duration arms (6, 8, 10, and 12 weeks) were double blinded, while the Griseofulvin active comparator arm was open-label (SFO327C T202). The objective of the study was to identify a safe and most appropriate treatment duration with terbinafine (tablets) in patients with Tinea capitis caused by Microsporum species. Dose administration of terbinafine was based on body weight in both studies as follows: <20 kg: 62.5 mg, 20-40 kg: 125 mg, >40 kg: 250 mg, given once daily. In both studies, terbinafine (Lamisil) was very well tolerated. Analysis of the efficacy data showed that both 2 and 4-week treatment duration provided good efficacy in T. capitis caused by Trichophyton species. In the Microsporum study, there was no significant difference in complete cure rates between the different treatment duration groups and 6-week treatment showed high complete cure rate (62%) with good tolerability and compliance. These results show that terbinafine (Lamisil) reduced treatment duration from 6-8 weeks to only 2-4 weeks in T. capitis caused by Trichophyton species compared to standard therapy with griseofulvin.
In phase II clinical studies conducted in Tinea capitis, adverse events reported from the 588 children enrolled were, in general, mild, relatively infrequent and often had an uncertain relationship to treatment. There were 11 reports of elevated SGPT levels and one of taste loss. Other events included mild gastrointestinal or skin symptoms, and laboratory findings indicative of intercurrent infections.
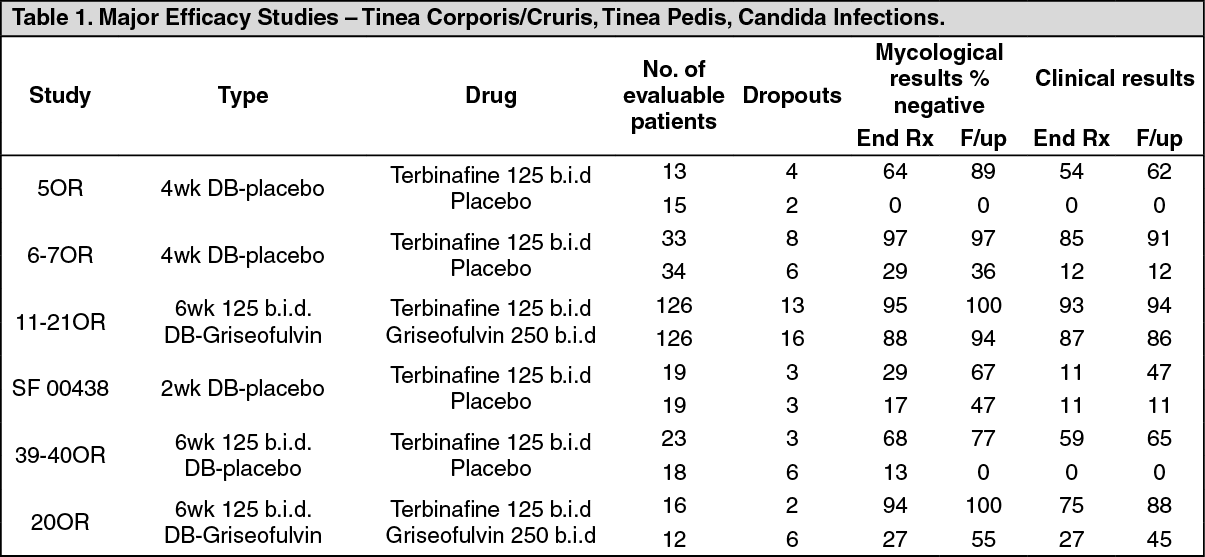
Fungal infections of the skin (tinea corporis, tinea cruris, tinea pedis) and yeast infections of the skin caused by the genus Candida (e.g. Candida albicans) where oral therapy is generally considered appropriate owing to the site, severity or extent of the infection: Three controlled, double blind, randomized, multicenter studies 5OR (4 week study), 6-7OR (4 week study) and 11-21OR (6 week study), evaluated efficacy and safety of Terbinafine (Lamisil) tablets in the treatment of Tinea corporis and cruris.
Two double blind, placebo controlled studies (85OR, 7OR) evaluated the efficacy of terbinafine (Lamisil) 125 mg b.i.d. in patients diagnosed with Tinea corporis/cruris. The studies included a total of 43 patients randomized to terbinafine (Lamisil) and 45 on placebo. There was no significant difference in terms of demographic and anamnestic data within groups. Efficacy, demonstrated by negative mycology tests and a reduction in clinical symptomatology, was evaluated at 4 weeks and at the follow-up examination. In both studies, minimal efficacy was demonstrated in patients treated with placebo compared to the efficacy of orally administered terbinafine at the end of therapy and at follow up.
The third study (11-21OR), a 6 weeks, double blind, randomized, multicenter study compared efficacy and safety of terbinafine (Lamisil) 125mg b.i.d. to griseofulvin 250mg b.i.d. One hundred twenty six (126) patients in each group were included in the efficacy analysis. This study showed high rate of mycological cure, reduction in signs and symptoms in the terbinafine treated study arm and significantly better (93-94%) overall efficacy at the end of therapy and at follow up of terbinafine 125 mg b.i.d. compared to 86-87% overall efficacy of comparator.
In summary, terbinafine (Lamisil) 125 mg b.i.d. administered for the period of 4-6 weeks demonstrated statistically superior efficacy compared to placebo and marketed drug griseofulvin in the treatment of Tinea corporis/cruris in the above major efficacy studies.
In a double blind, placebo controlled 4 weeks study SF 00438, terbinafine (Lamisil) 125 mg b.i.d was compared to placebo in patients with cutaneous candidiasis. Twenty two patients were randomized to each treatment arm, of which 19 were evaluated respectively. Of those, 29% of patients in the treatment arm and 17% of patients on placebo demonstrated mycological cure at the end of treatment and 67% of terbinafine-treated patients had negative mycological results at the end of follow up. Given the above response rates, 2 weeks therapy of terbinafine should be the minimum duration of treatment period and approximately half of the patients would require 3-4 weeks of treatment to achieve cure.
Two double blind, controlled studies compared terbinafine (Lamisil) 125 mg b.i.d. to placebo (39-40OR) and to griseofulvin 250 mg b.i.d. (20OR) in the treatment of Tinea pedis. Both studies recruited patients with chronic, recurrent disease. In the study 39-40OR, 65% of patients on terbinafine reported mycological cure at follow up whereas none of the placebo treated patients responded. In the study 20OR, terbinafine was shown to be highly effective with 88% of cure at follow up after 6 weeks therapy compared to 45% of patients on griseofulvin. These patients when observed after 10 months reported 94% cure rate, compared to 30% efficacy of griseofulvin in the same patient population. (See Table 1.)
Terbinafine interferes specifically with fungal sterol biosynthesis at an early step. This leads to a deficiency in ergosterol and to an intracellular accumulation of squalene, resulting in fungal cell death. Terbinafine acts by inhibition of squalene epoxidase in the fungal cell membrane. The enzyme squalene epoxidase is not linked to the cytochrome P450 system.
Pharmacodynamics: When given orally, terbinafine accumulates in skin, hair and nails at levels associated with fungicidal activity.
Clinical Studies: Onychomycosis: The efficacy of terbinafine (Lamisil) tablets in the treatment of onychomycosis is illustrated by the response of patients with toenail and/or fingernail infections who participated in three US/Canadian placebo-controlled clinical trials (SFD301, SF5 and SF1508).
Results of the first toenail study, as assessed at week 48 (12 weeks of treatment with 36 weeks follow-up after completion of therapy), demonstrated mycological cure, defined as simultaneous occurrence of negative KOH plus negative culture, in 70% of patients. Fifty-nine percent (59%) of patients experienced effective treatment (mycological cure plus 0% nail involvement or >5mm of new unaffected nail growth); 38% of patients demonstrated mycological cure plus clinical cure (0% nail involvement).
In a second toenail study of dermatophytic onychomycosis, in which non-dermatophytes were also cultured, similar efficacy against the dermatophytes was demonstrated. The pathogenic role of the non-dermatophytes cultured in the presence of dermatophytic onychomycosis has not been established. The clinical significance of this association is unknown.
Results of the fingernail study, as assessed at week 24 (6 weeks of treatment with 18 weeks follow-up after completion of therapy), demonstrated mycological cure in 79% of patients, effective treatment in 75% of the patients, and mycological cure plus clinical cure in 59% of the patients.
The mean time to treatment success for onychomycosis was approximately 10 months for the first toenail study and 4 months for the fingernail study. In the first toenail study, for patients evaluated at least six months after achieving clinical cure and at least one year after completing terbinafine (Lamisil) therapy, the clinical relapse rate was approximately 15%.
Tinea capitis: In the three comparative efficacy studies SF 8001, SFE 304, SF 8002 oral terbinafine (Lamisil) (62.5 - 250 mg daily) was given to a total of 117 evaluable patients, of whom over 97% were children. Single daily doses were given after the evening meal for 4 weeks (Terbinafine (Lamisil)) or 8 weeks (griseofulvin). Efficacy, demonstrated by negative mycology tests and a reduction in symptomatology, was evaluated at 8 weeks and at the follow-up examination (Week 12 for Studies SF 8001 and SFE 304, Week 24 for Study SF 8002). Negative mycology test results at follow-up were achieved by 85%, 88% and 72% of patients given terbinafine (Lamisil) in the three studies - the corresponding figures for griseofulvin were 73%, 89% and 69%. The derived variable "effective treatment" (negative mycology plus no, or only mild, symptoms and signs) was achieved in 82%, 78% and 69% of terbinafine-treated patients, compared with 66%, 74% and 59% in patients given griseofulvin; the difference was statistically significant in favor of terbinafine in Study SF 8001.
Two phase II treatment duration finding studies totaling 342 patients (mostly children) with T. capitis have been completed.
A 12-week randomized, double-blind, parallel group study was conducted in the United States and in Canada in children with Tinea capitis infection due to Trichophyton species (SFO327C T201). The objective of the study was to determine the optimal duration (1, 2 or 4 weeks) and safety of treatment with terbinafine (Lamisil) (tablets), given at weight adjusted doses once daily.
A second 16-week randomized, active-controlled, parallel-group, multicenter study was conducted in Europe in patients with Tinea capitis (>4 years) due to Microsporum species. The terbinafine (Lamisil) treatment duration arms (6, 8, 10, and 12 weeks) were double blinded, while the Griseofulvin active comparator arm was open-label (SFO327C T202). The objective of the study was to identify a safe and most appropriate treatment duration with terbinafine (tablets) in patients with Tinea capitis caused by Microsporum species. Dose administration of terbinafine was based on body weight in both studies as follows: <20 kg: 62.5 mg, 20-40 kg: 125 mg, >40 kg: 250 mg, given once daily. In both studies, terbinafine (Lamisil) was very well tolerated. Analysis of the efficacy data showed that both 2 and 4-week treatment duration provided good efficacy in T. capitis caused by Trichophyton species. In the Microsporum study, there was no significant difference in complete cure rates between the different treatment duration groups and 6-week treatment showed high complete cure rate (62%) with good tolerability and compliance. These results show that terbinafine (Lamisil) reduced treatment duration from 6-8 weeks to only 2-4 weeks in T. capitis caused by Trichophyton species compared to standard therapy with griseofulvin.
In phase II clinical studies conducted in Tinea capitis, adverse events reported from the 588 children enrolled were, in general, mild, relatively infrequent and often had an uncertain relationship to treatment. There were 11 reports of elevated SGPT levels and one of taste loss. Other events included mild gastrointestinal or skin symptoms, and laboratory findings indicative of intercurrent infections.
Fungal infections of the skin (tinea corporis, tinea cruris, tinea pedis) and yeast infections of the skin caused by the genus Candida (e.g. Candida albicans) where oral therapy is generally considered appropriate owing to the site, severity or extent of the infection: Three controlled, double blind, randomized, multicenter studies 5OR (4 week study), 6-7OR (4 week study) and 11-21OR (6 week study), evaluated efficacy and safety of Terbinafine (Lamisil) tablets in the treatment of Tinea corporis and cruris.
Two double blind, placebo controlled studies (85OR, 7OR) evaluated the efficacy of terbinafine (Lamisil) 125 mg b.i.d. in patients diagnosed with Tinea corporis/cruris. The studies included a total of 43 patients randomized to terbinafine (Lamisil) and 45 on placebo. There was no significant difference in terms of demographic and anamnestic data within groups. Efficacy, demonstrated by negative mycology tests and a reduction in clinical symptomatology, was evaluated at 4 weeks and at the follow-up examination. In both studies, minimal efficacy was demonstrated in patients treated with placebo compared to the efficacy of orally administered terbinafine at the end of therapy and at follow up.
The third study (11-21OR), a 6 weeks, double blind, randomized, multicenter study compared efficacy and safety of terbinafine (Lamisil) 125mg b.i.d. to griseofulvin 250mg b.i.d. One hundred twenty six (126) patients in each group were included in the efficacy analysis. This study showed high rate of mycological cure, reduction in signs and symptoms in the terbinafine treated study arm and significantly better (93-94%) overall efficacy at the end of therapy and at follow up of terbinafine 125 mg b.i.d. compared to 86-87% overall efficacy of comparator.
In summary, terbinafine (Lamisil) 125 mg b.i.d. administered for the period of 4-6 weeks demonstrated statistically superior efficacy compared to placebo and marketed drug griseofulvin in the treatment of Tinea corporis/cruris in the above major efficacy studies.
In a double blind, placebo controlled 4 weeks study SF 00438, terbinafine (Lamisil) 125 mg b.i.d was compared to placebo in patients with cutaneous candidiasis. Twenty two patients were randomized to each treatment arm, of which 19 were evaluated respectively. Of those, 29% of patients in the treatment arm and 17% of patients on placebo demonstrated mycological cure at the end of treatment and 67% of terbinafine-treated patients had negative mycological results at the end of follow up. Given the above response rates, 2 weeks therapy of terbinafine should be the minimum duration of treatment period and approximately half of the patients would require 3-4 weeks of treatment to achieve cure.
Two double blind, controlled studies compared terbinafine (Lamisil) 125 mg b.i.d. to placebo (39-40OR) and to griseofulvin 250 mg b.i.d. (20OR) in the treatment of Tinea pedis. Both studies recruited patients with chronic, recurrent disease. In the study 39-40OR, 65% of patients on terbinafine reported mycological cure at follow up whereas none of the placebo treated patients responded. In the study 20OR, terbinafine was shown to be highly effective with 88% of cure at follow up after 6 weeks therapy compared to 45% of patients on griseofulvin. These patients when observed after 10 months reported 94% cure rate, compared to 30% efficacy of griseofulvin in the same patient population. (See Table 1.)
Pharmacokinetics: Absorption: Following oral administration, terbinafine is well absorbed (>70%). A single oral dose of 250 mg terbinafine resulted in a mean peak plasma concentration of 1.3 microgram/mL within 1.5 hours of administration. At steady-state (70% steady state is achieved in approximately 28 days), in comparison to a single dose, peak concentration of terbinafine was on average 25% higher and plasma AUC increased by a factor of 2.3.
Distribution: Terbinafine binds strongly to plasma proteins (99%). It rapidly diffuses through the dermis and accumulates in the lipophilic stratum corneum. Terbinafine is also secreted in sebum, thus achieving high concentrations in hair follicles, hair and sebum-rich skin. There is also evidence that terbinafine is distributed into the nail plate within the first few weeks after commencing therapy.
Biotransformation/Metabolism: Terbinafine is metabolized rapidly and extensively by at least seven CYP isoenzymes with major contributions from CYP2C9, CYP1A2, CYP3A4, CYP2C8 and CYP2C19. Biotransformation results in metabolites with no antifungal activity.
Elimination: The metabolites are excreted predominantly in the urine. From the increase in plasma AUC at steady state an effective half-life of Yulex30 hours was calculated. Multiple dose administration followed by extended blood sampling revealed a triphasic elimination with a terminal half-life of approximately 16.5 days.
Bioavailability: The absolute bioavailability of terbinafine from Lamisil tablets as a result of first-pass metabolism is approximately 50%.
Special populations: No clinically relevant age-dependent changes in steady-state plasma concentrations of terbinafine have been observed.
Single dose pharmacokinetic studies in patients with renal impairment (creatinine clearance <50 mL/min) or with pre-existing liver disease have shown that the clearance of terbinafine (Lamisil) tablets may be reduced by about 50%.
Toxicology: Non-Clinical Safety Data: In long-term studies (up to 1 year) in rats and dogs no marked toxic effects were seen in either species up to oral doses of about 100 mg/kg a day. At high oral doses, the liver and possibly also the kidneys were identified as potential target organs.
In a two-year oral carcinogenicity study in mice, no neoplastic or other abnormal findings attributable to treatment were made up to doses of 130 (males) and 156 (females) mg/kg a day. In a two-year oral carcinogenicity study in rats, an increased incidence of liver tumors was observed in males at the highest dose level of 69 mg/kg a day. The changes, which may be associated with peroxisome proliferation have been shown to be species-specific since they were not seen in the carcinogenicity study in mice or in other studies in mice, dogs or monkeys.
During high-dose studies in monkeys, refractile irregularities were observed in the retina at the higher doses (non-toxic effect level 50 mg/kg). These irregularities were associated with the presence of a terbinafine metabolite in ocular tissue and disappeared after drug discontinuation. They were not associated with histological changes.
An 8-week oral study in juvenile rats provided a no-toxic-effect level (NTEL) of close to 100 mg/kg/day, with the only finding being slightly increased liver weights, while in maturing dogs at ≥100 mg/kg/day (AUC values about 13x (m) and 6x (f) those in children), signs of central nervous system (CNS) disturbance including single episodes of convulsions in individual animals were observed. Similar findings have been observed at high systemic exposure upon intravenous administration of terbinafine to adult rats or monkeys.
A standard battery of in vitro and in vivo genotoxicity tests revealed no evidence of mutagenic or clastogenic potential.
No adverse effects on fertility or other reproduction parameters were observed in studies in rats or rabbits.
MedsGo Class
Antifungals
Features
Dosage
250 mg
Ingredients
- Terbinafine
Packaging
Tablet 1's
Generic Name
Terbinafine
Registration Number
DR-XY15999
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions


