Indications/Uses
For the treatment of adults, adolescents and children 2 years or above with diabetes mellitus, where treatment with insulin is required.
Dosage/Direction for Use
General: Insulin glargine is a novel recombinant human insulin analogue, equipotent to human insulin. It exhibits a peakless glucose-lowering profile with a prolonged duration of action.
Insulin glargine (Podevta) is given subcutaneously once a day. It may be administered at any time during the day, however, at the same time every day.
The desired blood glucose levels as well as the doses and timing of anti-diabetic medications must be determined and adjusted individually.
Dose adjustment may be required, for example, if the patient's weight, life-style changes, change in timing of insulin dose or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia (see Precautions). Any change of insulin dose should be made cautiously and only under medical supervision.
Insulin glargine (Podevta) is not the insulin of choice for the treatment of diabetic ketoacidosis. An intravenous, short-acting insulin is the preferred treatment.
In basal bolus injection regimens, usually 40 to 60 % of the daily dose is administered as insulin glargine to cover basal insulin requirements.
In a clinical study with patients with type 2 diabetes on oral anti-diabetic agents, combination therapy was started with a dose of 10 IU insulin glargine once daily and the treatment regimen subsequently adjusted individually.
Blood glucose monitoring is recommended for all patients with diabetes.
Change-over to Insulin glargine (Podevta): When changing from a treatment regimen with an intermediate or another long-acting insulin to a regimen with Insulin glargine (Podevta), the amount and timing of short-acting insulin or fast-acting insulin analogue or of the dose of any oral anti-diabetic drug may need to be adjusted.
In clinical studies when patients were transferred from once daily NPH or ultralente insulin to once daily Insulin glargine (Podevta), the initial dose was usually not changed (i.e. amount of International Units, IU, of Insulin glargine (Podevta) per day equal to IU of NPH insulin). In studies when patients were transferred from twice daily NPH insulin to once daily Insulin glargine (Podevta) at bedtime, to reduce the risk of hypoglycaemia, the initial dose (IU), was usually reduced by approximately 20% (compared to total daily IU of NPH insulin) and then adjusted based on patient response.
A program of close metabolic monitoring under medical supervision is recommended during transfer and in the initial weeks thereafter. As with all insulin analogues, this is particularly true for patients which, due to antibodies to human insulin, need high insulin doses and may experience a markedly improved insulin response with insulin glargine.
With improved metabolic control and resultant increase in insulin sensitivity (reduced insulin requirements) further adjustment of the doses of Insulin glargine (Podevta) and other insulins or oral anti-diabetic drugs in the regimen may become necessary.
Mixing, diluting: Insulin glargine (Podevta) must not be mixed with any other insulin. Mixing can change the time/action profile of Insulin glargine (Podevta) and cause precipitation.
Insulin glargine (Podevta) must not be diluted. Diluting can change the time/action profile of Insulin glargine (Podevta).
Special Populations: Children: Insulin glargine (Podevta) can be administrated to children ≥1 year of age.
Administration to children < 1 year has not been studied.
Elderly: In elderly patients with diabetes, it is recommended that the initial dosing, dose increments, and maintenance dosage be conservative to avoid hypoglycaemic reactions. Hypoglycaemia may be difficult to recognise in the elderly (See Precautions).
Administration: Insulin glargine (Podevta) is administered by subcutaneous tissue injection.
Insulin glargine (Podevta) is not intended for intravenous administration.
The prolonged duration of activity of insulin glargine is dependent on injection into the subcutaneous space. Intravenous administration of the usual subcutaneous dose could result in severe hypoglycaemia.
As with all insulins, injection sites within an injection area (abdomen, thigh or deltoid) must be rotated from one injection to the next.
Absorption of insulin glargine is not different between abdominal, thigh or deltoid subcutaneous injection area. As for all insulins, the rate of absorption and consequently the onset and duration of action may be affected by exercise and other variables.
Insulin glargine (Podevta) is a clear solution, not a suspension. As such it does not require resuspension before use.
Cartridge or Cartridge system version only: If the pen malfunctions, Insulin glargine (Podevta) may be drawn from the cartridge or cartridge system into a syringe (suitable for an insulin with 100 IU/mL) and injected.
The syringes must not contain any other medicinal product or residue.
Insulin glargine (Podevta) is given subcutaneously once a day. It may be administered at any time during the day, however, at the same time every day.
The desired blood glucose levels as well as the doses and timing of anti-diabetic medications must be determined and adjusted individually.
Dose adjustment may be required, for example, if the patient's weight, life-style changes, change in timing of insulin dose or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia (see Precautions). Any change of insulin dose should be made cautiously and only under medical supervision.
Insulin glargine (Podevta) is not the insulin of choice for the treatment of diabetic ketoacidosis. An intravenous, short-acting insulin is the preferred treatment.
In basal bolus injection regimens, usually 40 to 60 % of the daily dose is administered as insulin glargine to cover basal insulin requirements.
In a clinical study with patients with type 2 diabetes on oral anti-diabetic agents, combination therapy was started with a dose of 10 IU insulin glargine once daily and the treatment regimen subsequently adjusted individually.
Blood glucose monitoring is recommended for all patients with diabetes.
Change-over to Insulin glargine (Podevta): When changing from a treatment regimen with an intermediate or another long-acting insulin to a regimen with Insulin glargine (Podevta), the amount and timing of short-acting insulin or fast-acting insulin analogue or of the dose of any oral anti-diabetic drug may need to be adjusted.
In clinical studies when patients were transferred from once daily NPH or ultralente insulin to once daily Insulin glargine (Podevta), the initial dose was usually not changed (i.e. amount of International Units, IU, of Insulin glargine (Podevta) per day equal to IU of NPH insulin). In studies when patients were transferred from twice daily NPH insulin to once daily Insulin glargine (Podevta) at bedtime, to reduce the risk of hypoglycaemia, the initial dose (IU), was usually reduced by approximately 20% (compared to total daily IU of NPH insulin) and then adjusted based on patient response.
A program of close metabolic monitoring under medical supervision is recommended during transfer and in the initial weeks thereafter. As with all insulin analogues, this is particularly true for patients which, due to antibodies to human insulin, need high insulin doses and may experience a markedly improved insulin response with insulin glargine.
With improved metabolic control and resultant increase in insulin sensitivity (reduced insulin requirements) further adjustment of the doses of Insulin glargine (Podevta) and other insulins or oral anti-diabetic drugs in the regimen may become necessary.
Mixing, diluting: Insulin glargine (Podevta) must not be mixed with any other insulin. Mixing can change the time/action profile of Insulin glargine (Podevta) and cause precipitation.
Insulin glargine (Podevta) must not be diluted. Diluting can change the time/action profile of Insulin glargine (Podevta).
Special Populations: Children: Insulin glargine (Podevta) can be administrated to children ≥1 year of age.
Administration to children < 1 year has not been studied.
Elderly: In elderly patients with diabetes, it is recommended that the initial dosing, dose increments, and maintenance dosage be conservative to avoid hypoglycaemic reactions. Hypoglycaemia may be difficult to recognise in the elderly (See Precautions).
Administration: Insulin glargine (Podevta) is administered by subcutaneous tissue injection.
Insulin glargine (Podevta) is not intended for intravenous administration.
The prolonged duration of activity of insulin glargine is dependent on injection into the subcutaneous space. Intravenous administration of the usual subcutaneous dose could result in severe hypoglycaemia.
As with all insulins, injection sites within an injection area (abdomen, thigh or deltoid) must be rotated from one injection to the next.
Absorption of insulin glargine is not different between abdominal, thigh or deltoid subcutaneous injection area. As for all insulins, the rate of absorption and consequently the onset and duration of action may be affected by exercise and other variables.
Insulin glargine (Podevta) is a clear solution, not a suspension. As such it does not require resuspension before use.
Cartridge or Cartridge system version only: If the pen malfunctions, Insulin glargine (Podevta) may be drawn from the cartridge or cartridge system into a syringe (suitable for an insulin with 100 IU/mL) and injected.
The syringes must not contain any other medicinal product or residue.
Overdosage
Signs and Symptoms: An excess of insulin, relative to food intake, energy expenditure or both, may lead to severe and sometimes prolonged and life-threatening hypoglycaemia.
Management: Mild episodes of hypoglycaemia can usually be treated with oral carbohydrates. Adjustments in drug dosage, meal patterns, or exercise may be needed.
More severe episodes culminating in coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycaemia may recur after apparent clinical recovery.
Management: Mild episodes of hypoglycaemia can usually be treated with oral carbohydrates. Adjustments in drug dosage, meal patterns, or exercise may be needed.
More severe episodes culminating in coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycaemia may recur after apparent clinical recovery.
Contraindications
Insulin glargine (Podevta) must not be used in patients hypersensitive to insulin glargine or any of the excipients.
Special Precautions
General: Insulin therapy generally requires appropriate diabetes self-management skills, including glucose monitoring, proper injection technique, and hypo- and hyperglycaemia management. Patients should be instructed on such self-management procedures. Additionally, patients must be instructed on handling of special situations such as an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake or skipped meals. The extent of patient participation in his/her diabetes management is variable and is generally determined by the physician.
Insulin treatment requires constant alertness to the possibility of hyper- and hypoglycaemia.
Patients and their relatives must know what steps to take if hyperglycaemia or hypoglycaemia occurs or is suspected, and they must know when to inform a physician.
In case of insufficient glucose control or a tendency to hyper- or hypoglycaemic episodes, patient's compliance with the prescribed insulin regimen, injection sites and proper injection techniques, the handling of injection devices and all other relevant factors must to be reviewed before dose adjustment is considered.
Hypoglycaemia: The time of occurrence of hypoglycaemia depends on the action profile of the insulins used and may, therefore, change when the treatment regimen is changed.
As with all insulins, particular caution should be exercised, and intensified blood glucose monitoring is advisable, in patients in whom sequelae of hypoglycaemic episodes might be of particular clinical relevance. For example these could be patients with significant stenoses of the coronary arteries or of the blood vessels supplying the brain (risk of cardiac or cerebral complications of hypoglycaemia) as well as patients with proliferative retinopathy, particularly if not treated with photocoagulation (risk of transient amaurosis following hypoglycaemia).
In a clinical study, symptoms of hypoglycaemia or counter-regulatory hormone responses were similar after intravenous insulin glargine and human insulin both in healthy volunteers and patients with type 1 diabetes.
However, under certain conditions, as with all insulins, the warning symptoms of hypoglycaemia maybe changed, be less pronounced or absent, for example: if glycaemic control is markedly improved; if hypoglycaemia is developing gradually; in elderly patients; where an autonomic neuropathy is present; in patients with a long history of diabetes; in patients suffering from a psychiatric illness; in patients receiving concurrent treatment with certain other drugs (see Interactions).
Such situations may result in severe hypoglycaemia (and possibly, loss of consciousness) prior to patient's awareness of hypoglycaemia.
The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia.
If normal or decreased values for glycated haemoglobin are noted, the possibility of recurrent, unrecognised (especially nocturnal) episodes of hypoglycaemia must be considered.
Compliance of the patient with the dosage and dietary regimen, correct insulin administration and awareness of hypoglycaemia symptoms are essential to reduce the risk of hypoglycaemia.
Presence of factors which increase the susceptibility to hypoglycaemia requires particularly close monitoring and may necessitate dose adjustment include: change in the injection area; increase of insulin sensitivity (e.g. by removal of stress factors); unaccustomed, increased or prolonged physical exercise; intercurrent illness (e.g. vomiting, diarrhoea); inadequate food intake; alcohol consumption; certain uncompensated endocrine disorders; concomitant treatment with certain medications.
In patients with renal impairment, insulin requirements may be diminished due to reduced insulin metabolism. In the elderly, progressive deterioration of renal function may lead to steady decrease in insulin requirements.
In patients with severe hepatic impairment, insulin requirements may be diminished due to reduced capacity for gluconeogenesis and reduced insulin metabolism.
Hypoglycaemia can generally be corrected by immediate carbohydrate intake. So that initial corrective action can be taken immediately, patients must carry a minimum of 20 grams of carbohydrates with them at all times.
Intercurrent illness: Intercurrent illness requires intensified metabolic monitoring. In many cases urine tests for ketones are indicated, and often it is necessary to adjust the insulin dose. The insulin requirement is often increased. In patients with type 1 diabetes, carbohydrate supplies must be maintained even if patients are able to eat only little or no food, or are vomiting etc.; in patients with type 1 diabetes insulin must never be omitted entirely.
Driving a Vehicle or Performing Other Hazardous Tasks: The patient's ability to concentrate and react may be impaired as a result of, for example, hypoglycaemia or hyperglycaemia or, for example, as a result of visual impairment. This may constitute a risk in situations where these abilities are of special importance (e.g. driving a car or operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is particularly important in those who have reduced or absent awareness of the warning symptoms of hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be considered in these circumstances.
Insulin treatment requires constant alertness to the possibility of hyper- and hypoglycaemia.
Patients and their relatives must know what steps to take if hyperglycaemia or hypoglycaemia occurs or is suspected, and they must know when to inform a physician.
In case of insufficient glucose control or a tendency to hyper- or hypoglycaemic episodes, patient's compliance with the prescribed insulin regimen, injection sites and proper injection techniques, the handling of injection devices and all other relevant factors must to be reviewed before dose adjustment is considered.
Hypoglycaemia: The time of occurrence of hypoglycaemia depends on the action profile of the insulins used and may, therefore, change when the treatment regimen is changed.
As with all insulins, particular caution should be exercised, and intensified blood glucose monitoring is advisable, in patients in whom sequelae of hypoglycaemic episodes might be of particular clinical relevance. For example these could be patients with significant stenoses of the coronary arteries or of the blood vessels supplying the brain (risk of cardiac or cerebral complications of hypoglycaemia) as well as patients with proliferative retinopathy, particularly if not treated with photocoagulation (risk of transient amaurosis following hypoglycaemia).
In a clinical study, symptoms of hypoglycaemia or counter-regulatory hormone responses were similar after intravenous insulin glargine and human insulin both in healthy volunteers and patients with type 1 diabetes.
However, under certain conditions, as with all insulins, the warning symptoms of hypoglycaemia maybe changed, be less pronounced or absent, for example: if glycaemic control is markedly improved; if hypoglycaemia is developing gradually; in elderly patients; where an autonomic neuropathy is present; in patients with a long history of diabetes; in patients suffering from a psychiatric illness; in patients receiving concurrent treatment with certain other drugs (see Interactions).
Such situations may result in severe hypoglycaemia (and possibly, loss of consciousness) prior to patient's awareness of hypoglycaemia.
The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia.
If normal or decreased values for glycated haemoglobin are noted, the possibility of recurrent, unrecognised (especially nocturnal) episodes of hypoglycaemia must be considered.
Compliance of the patient with the dosage and dietary regimen, correct insulin administration and awareness of hypoglycaemia symptoms are essential to reduce the risk of hypoglycaemia.
Presence of factors which increase the susceptibility to hypoglycaemia requires particularly close monitoring and may necessitate dose adjustment include: change in the injection area; increase of insulin sensitivity (e.g. by removal of stress factors); unaccustomed, increased or prolonged physical exercise; intercurrent illness (e.g. vomiting, diarrhoea); inadequate food intake; alcohol consumption; certain uncompensated endocrine disorders; concomitant treatment with certain medications.
In patients with renal impairment, insulin requirements may be diminished due to reduced insulin metabolism. In the elderly, progressive deterioration of renal function may lead to steady decrease in insulin requirements.
In patients with severe hepatic impairment, insulin requirements may be diminished due to reduced capacity for gluconeogenesis and reduced insulin metabolism.
Hypoglycaemia can generally be corrected by immediate carbohydrate intake. So that initial corrective action can be taken immediately, patients must carry a minimum of 20 grams of carbohydrates with them at all times.
Intercurrent illness: Intercurrent illness requires intensified metabolic monitoring. In many cases urine tests for ketones are indicated, and often it is necessary to adjust the insulin dose. The insulin requirement is often increased. In patients with type 1 diabetes, carbohydrate supplies must be maintained even if patients are able to eat only little or no food, or are vomiting etc.; in patients with type 1 diabetes insulin must never be omitted entirely.
Driving a Vehicle or Performing Other Hazardous Tasks: The patient's ability to concentrate and react may be impaired as a result of, for example, hypoglycaemia or hyperglycaemia or, for example, as a result of visual impairment. This may constitute a risk in situations where these abilities are of special importance (e.g. driving a car or operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is particularly important in those who have reduced or absent awareness of the warning symptoms of hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be considered in these circumstances.
Use In Pregnancy & Lactation
Use in Pregnancy: There are no randomized controlled clinical studies of the use of insulin glargine in pregnant women.
A large number (more than 1000 retrospective and prospective pregnancy outcomes) of exposed pregnancies from Post Marketing Surveillance indicate no specific adverse effects of insulin glargine on pregnancy or on the health of the foetus and newborn child.
Furthermore, a meta-analysis of eight observational clinical studies including 331 women using insulin glargine and 371 women using insulin NPH was performed to assess the safety of insulin glargine and insulin NPH in gestational or pregestational diabetes. No significant differences in safety-related maternal or neonatal outcomes were seen between insulin glargine and insulin NPH during pregnancy.
Animal studies, with doses up to 6 to 40 times the human doses, do not indicate direct harmful effects on the pregnancy.
It is essential for patients with pre-existing or gestational diabetes to maintain good metabolic control throughout pregnancy to prevent adverse outcomes associated with hyperglycemia.
Insulin glargine (Podevta) can be used during pregnancy, if clinically needed.
Insulin requirements may decrease during the first trimester and generally increase during the second and third trimesters. Immediately after delivery, insulin requirements decline rapidly.
Careful monitoring of glucose control, is essential in such patients.
Patients with diabetes must inform their doctor if they are pregnant or are contemplating pregnancy.
Use in Lactation: Lactating women may require adjustments in insulin dose and diet.
A large number (more than 1000 retrospective and prospective pregnancy outcomes) of exposed pregnancies from Post Marketing Surveillance indicate no specific adverse effects of insulin glargine on pregnancy or on the health of the foetus and newborn child.
Furthermore, a meta-analysis of eight observational clinical studies including 331 women using insulin glargine and 371 women using insulin NPH was performed to assess the safety of insulin glargine and insulin NPH in gestational or pregestational diabetes. No significant differences in safety-related maternal or neonatal outcomes were seen between insulin glargine and insulin NPH during pregnancy.
Animal studies, with doses up to 6 to 40 times the human doses, do not indicate direct harmful effects on the pregnancy.
It is essential for patients with pre-existing or gestational diabetes to maintain good metabolic control throughout pregnancy to prevent adverse outcomes associated with hyperglycemia.
Insulin glargine (Podevta) can be used during pregnancy, if clinically needed.
Insulin requirements may decrease during the first trimester and generally increase during the second and third trimesters. Immediately after delivery, insulin requirements decline rapidly.
Careful monitoring of glucose control, is essential in such patients.
Patients with diabetes must inform their doctor if they are pregnant or are contemplating pregnancy.
Use in Lactation: Lactating women may require adjustments in insulin dose and diet.
Adverse Reactions
The following CIOMS frequency rating is used, when applicable: Very common ≥10%; Common ≥1 and <10%; Uncommon ≥0.1 and <1%; Rare ≥0.01 and <0.1%; Very rare <0.01%, Unknown (cannot be estimated from available data).
Hypoglycaemia: Hypoglycaemia, in general the most frequent adverse reaction of insulin therapy, may occur if the insulin dose is too high in relation to the insulin requirement.
As with all insulins, severe hypoglycaemic attacks, especially if recurrent, may lead to neurological damage. Prolonged or severe hypoglycaemic episodes may be life-threatening.
In many patients, the signs and symptoms of neuroglycopenia are preceded by signs of adrenergic counter regulation.
Generally, the greater and more rapid the decline in blood glucose, the more marked is the phenomenon of counter-regulation and its symptoms.
For hypoglycaemia incidences from clinical trials, see tables in section Pharmacology: Pharmacodynamics under Actions.
Eyes: A marked change in glycaemic control may cause temporary visual impairment, due to temporary alteration in the turgidity and refractive index of the lens.
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, as for all insulin regimens, intensification of insulin therapy with abrupt improvement in glycaemic control may be associated with temporary worsening of diabetic retinopathy.
In patients with proliferative retinopathy, particularly if not treated with photocoagulation, severe hypoglycaemic episodes may result in transient amaurosis.
See Pharmacology: Pharmacodynamics under Actions for additional information regarding retinopathy study results.
Lipodystrophy: Lipodystrophy, as with any insulin therapy, may occur at the injection site and delay insulin absorption.
In clinical studies, in regimens, which included insulin glargine, lipohypertrophy was observed in 1 to 2 % of patients, whereas lipoatrophy was uncommon. Continuous rotation of the injection site within a given area may help to reduce or prevent these reactions.
Injection site and allergic reactions: In clinical studies, using regimens, which included insulin glargine, injection site reactions were observed in 3 to 4 % of patients. As with any insulin therapy, such reactions include redness, pain, itching, hives, swelling, and inflammation. Most minor reactions to insulins usually resolve in a few days to a few weeks.
Immediate-type allergic reactions are rare. Such reactions to insulin (including insulin glargine) or the excipients may, for example, be associated with generalised skin reactions, angiooedema, bronchospasm, and hypotension and shock, and may be life threatening.
Other reactions: Insulin administration may cause insulin antibodies to form. In clinical studies, antibodies that cross react with human insulin and insulin glargine were observed in both NPH and insulin glargine treatment groups with similar incidences. In rare cases, the presence of such insulin antibodies may necessitate adjustment of the insulin dose in order to correct a tendency to hyperglycaemia or hypoglycaemia.
Insulin may cause, in rare cases, sodium retention and oedema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
Medication errors have been reported in which other insulins, particularly short-acting insulins, have been accidentally administered instead of insulin glargine.
Paediatric population: The safety profile for patients ≤18 years of age is similar to the safety profile for patients >18 years. No clinical study safety data are available in patients below 2 years of age.
Hypoglycaemia: Hypoglycaemia, in general the most frequent adverse reaction of insulin therapy, may occur if the insulin dose is too high in relation to the insulin requirement.
As with all insulins, severe hypoglycaemic attacks, especially if recurrent, may lead to neurological damage. Prolonged or severe hypoglycaemic episodes may be life-threatening.
In many patients, the signs and symptoms of neuroglycopenia are preceded by signs of adrenergic counter regulation.
Generally, the greater and more rapid the decline in blood glucose, the more marked is the phenomenon of counter-regulation and its symptoms.
For hypoglycaemia incidences from clinical trials, see tables in section Pharmacology: Pharmacodynamics under Actions.
Eyes: A marked change in glycaemic control may cause temporary visual impairment, due to temporary alteration in the turgidity and refractive index of the lens.
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, as for all insulin regimens, intensification of insulin therapy with abrupt improvement in glycaemic control may be associated with temporary worsening of diabetic retinopathy.
In patients with proliferative retinopathy, particularly if not treated with photocoagulation, severe hypoglycaemic episodes may result in transient amaurosis.
See Pharmacology: Pharmacodynamics under Actions for additional information regarding retinopathy study results.
Lipodystrophy: Lipodystrophy, as with any insulin therapy, may occur at the injection site and delay insulin absorption.
In clinical studies, in regimens, which included insulin glargine, lipohypertrophy was observed in 1 to 2 % of patients, whereas lipoatrophy was uncommon. Continuous rotation of the injection site within a given area may help to reduce or prevent these reactions.
Injection site and allergic reactions: In clinical studies, using regimens, which included insulin glargine, injection site reactions were observed in 3 to 4 % of patients. As with any insulin therapy, such reactions include redness, pain, itching, hives, swelling, and inflammation. Most minor reactions to insulins usually resolve in a few days to a few weeks.
Immediate-type allergic reactions are rare. Such reactions to insulin (including insulin glargine) or the excipients may, for example, be associated with generalised skin reactions, angiooedema, bronchospasm, and hypotension and shock, and may be life threatening.
Other reactions: Insulin administration may cause insulin antibodies to form. In clinical studies, antibodies that cross react with human insulin and insulin glargine were observed in both NPH and insulin glargine treatment groups with similar incidences. In rare cases, the presence of such insulin antibodies may necessitate adjustment of the insulin dose in order to correct a tendency to hyperglycaemia or hypoglycaemia.
Insulin may cause, in rare cases, sodium retention and oedema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
Medication errors have been reported in which other insulins, particularly short-acting insulins, have been accidentally administered instead of insulin glargine.
Paediatric population: The safety profile for patients ≤18 years of age is similar to the safety profile for patients >18 years. No clinical study safety data are available in patients below 2 years of age.
Drug Interactions
A number of substances affect glucose metabolism and may require insulin dose adjustment and particularly close monitoring.
The following are examples of substances that may increase the blood glucose lowering effect and susceptibility to hypoglycaemia: Oral anti-diabetic products, ACE inhibitors, salicylates, disopyramide; fibrates; fluoxetine, MAO inhibitors; pentoxifylline; propoxyphene; sulfonamide antibiotics.
The following are examples of substances that may reduce the blood glucose lowering effect: Corticosteroids; danazol; diazoxide; diuretics; sympathomimetic agents (such as epinephrine, salbutamol, terbutaline); glucagon; isoniazid; phenothiazine derivatives; somatropin; thyroid hormones; estrogens, progestogens (e.g. in oral contraceptives), protease inhibitors and atypical antipsychotic medications (e.g. olanzapine and clozapine).
Beta-blockers, clonidine, lithium salts and alcohol may either potentiate or weaken the blood glucose lowering effect of insulin. Pentamidine may cause hypoglycaemia, which may sometimes be followed by hyperglycaemia.
In addition, under the influence of sympatholytic medicinal products such as beta-blockers, clonidine, guanethidine and reserpine, the signs of adrenergic counter-regulation may be reduced or absent.
The following are examples of substances that may increase the blood glucose lowering effect and susceptibility to hypoglycaemia: Oral anti-diabetic products, ACE inhibitors, salicylates, disopyramide; fibrates; fluoxetine, MAO inhibitors; pentoxifylline; propoxyphene; sulfonamide antibiotics.
The following are examples of substances that may reduce the blood glucose lowering effect: Corticosteroids; danazol; diazoxide; diuretics; sympathomimetic agents (such as epinephrine, salbutamol, terbutaline); glucagon; isoniazid; phenothiazine derivatives; somatropin; thyroid hormones; estrogens, progestogens (e.g. in oral contraceptives), protease inhibitors and atypical antipsychotic medications (e.g. olanzapine and clozapine).
Beta-blockers, clonidine, lithium salts and alcohol may either potentiate or weaken the blood glucose lowering effect of insulin. Pentamidine may cause hypoglycaemia, which may sometimes be followed by hyperglycaemia.
In addition, under the influence of sympatholytic medicinal products such as beta-blockers, clonidine, guanethidine and reserpine, the signs of adrenergic counter-regulation may be reduced or absent.
Caution For Usage
Special Precautions for Disposal and Other Handling: Before first use, the pen must be stored at room temperature for 1 to 2 hours.
Inspect the cartridge before use. It must only be used if the solution is clear, colorless, with no solid particles visible, and if it is of water-like consistency. Since Insulin glargine (Podevta) is a solution, it does not require resuspension before use.
Insulin glargine (Podevta) must not be mixed with any other insulin or diluted. Mixing or diluting can change its time/action profile and mixing can cause precipitation.
Empty pens must never be reused and must be properly discarded. To prevent the possible transmission of disease, each pen must be used by one patient only.
Insulin label must always be checked before each injection to avoid medication errors between insulin glargine and other insulins (see Precautions).
Inspect the cartridge before use. It must only be used if the solution is clear, colorless, with no solid particles visible, and if it is of water-like consistency. Since Insulin glargine (Podevta) is a solution, it does not require resuspension before use.
Insulin glargine (Podevta) must not be mixed with any other insulin or diluted. Mixing or diluting can change its time/action profile and mixing can cause precipitation.
Empty pens must never be reused and must be properly discarded. To prevent the possible transmission of disease, each pen must be used by one patient only.
Insulin label must always be checked before each injection to avoid medication errors between insulin glargine and other insulins (see Precautions).
Storage
Not in-use pens: Store in a refrigerator (2°C-8°C).
Do not freeze.
Do not put Insulin glargine (Podevta) next to the freezer compartment or a freezer pack.
Keep the pre-filled pen in the outer carton in order to protect from light.
In use pens: The medicinal product may be stored for a maximum of 4 weeks not above 30°C and away from direct heat or direct light. Pens in use must not be stored in the refrigerator. The pen cap must be put back on the pen after each injection in order to protect from light.
Do not freeze.
Do not put Insulin glargine (Podevta) next to the freezer compartment or a freezer pack.
Keep the pre-filled pen in the outer carton in order to protect from light.
In use pens: The medicinal product may be stored for a maximum of 4 weeks not above 30°C and away from direct heat or direct light. Pens in use must not be stored in the refrigerator. The pen cap must be put back on the pen after each injection in order to protect from light.
Action
Pharmacology: Pharmacodynamics: Mechanism of Action/Pharmacodynamic Characteristics: Insulin glargine is a human insulin analogue designed to have a low solubility at neutral pH.
At pH 4 (as in the Insulin glargine injection solution), it is completely soluble.
After injection into the subcutaneous tissue, the acidic solution is neutralised leading to formation of micro-precipitates from which small amounts of insulin glargine are continuously released, providing a smooth, peakless, predictable concentration/time prole with a prolonged duration of action.
Insulin glargine is metabolised into 2 active metabolites M1 and M2 (see Pharmacokinetics as follows).
Insulin receptor binding: In vitro studies indicate that the affinity of insulin glargine and its metabolites M1 and M2 for the human insulin receptor is similar to the one of human insulin.
IGF-1 receptor binding: The affinity of insulin glargine for the human IGF-1 receptor is approximately 5 to 8-fold greater than that of human insulin (but approximately 70 to 80-fold lower than the one of IGF-1), whereas M1 and M2 bind the IGF-1 receptor with slightly lower affinity compared to human insulin.
The total therapeutic insulin concentration (insulin glargine and its metabolites) found in type 1 diabetic patients was markedly lower than what would be required for a half maximal occupation of the IGF-1 receptor and the subsequent activation of the mitogenic-proliferative pathway initiated by the IGF-1 receptor. Physiological concentrations of endogenous IGF-1 may activate the mitogenic-proliferative pathway; however, the therapeutic concentrations found in insulin therapy, including in Insulin glargine therapy, are considerably lower than the pharmacological concentrations required to activate the IGF-1 pathway.
The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism.
Insulin and its analogues lower blood glucose levels by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis in the adipocyte, inhibits proteolysis and enhances protein synthesis.
In clinical pharmacology studies, intravenous use of insulin glargine and human insulin have been shown to be equipotent when given at the same doses.
In euglycaemic clamp studies in healthy subjects or in patients with type 1 diabetes, the onset of action of subcutaneous insulin glargine was slower than with human NPH insulin, its effect profile was smooth and peakless, and the duration of its effect was prolonged. The following graph shows results from a study in patients. The median time between injection of the drug and the end of its pharmacological effect was 14.5 hours for NPH insulin while the median time for insulin glargine was 24 hours. The majority of patients on insulin glargine were still showing a response at this point of time, indicating an even longer duration of action. (See Figure.)
At pH 4 (as in the Insulin glargine injection solution), it is completely soluble.
After injection into the subcutaneous tissue, the acidic solution is neutralised leading to formation of micro-precipitates from which small amounts of insulin glargine are continuously released, providing a smooth, peakless, predictable concentration/time prole with a prolonged duration of action.
Insulin glargine is metabolised into 2 active metabolites M1 and M2 (see Pharmacokinetics as follows).
Insulin receptor binding: In vitro studies indicate that the affinity of insulin glargine and its metabolites M1 and M2 for the human insulin receptor is similar to the one of human insulin.
IGF-1 receptor binding: The affinity of insulin glargine for the human IGF-1 receptor is approximately 5 to 8-fold greater than that of human insulin (but approximately 70 to 80-fold lower than the one of IGF-1), whereas M1 and M2 bind the IGF-1 receptor with slightly lower affinity compared to human insulin.
The total therapeutic insulin concentration (insulin glargine and its metabolites) found in type 1 diabetic patients was markedly lower than what would be required for a half maximal occupation of the IGF-1 receptor and the subsequent activation of the mitogenic-proliferative pathway initiated by the IGF-1 receptor. Physiological concentrations of endogenous IGF-1 may activate the mitogenic-proliferative pathway; however, the therapeutic concentrations found in insulin therapy, including in Insulin glargine therapy, are considerably lower than the pharmacological concentrations required to activate the IGF-1 pathway.
The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism.
Insulin and its analogues lower blood glucose levels by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis in the adipocyte, inhibits proteolysis and enhances protein synthesis.
In clinical pharmacology studies, intravenous use of insulin glargine and human insulin have been shown to be equipotent when given at the same doses.
In euglycaemic clamp studies in healthy subjects or in patients with type 1 diabetes, the onset of action of subcutaneous insulin glargine was slower than with human NPH insulin, its effect profile was smooth and peakless, and the duration of its effect was prolonged. The following graph shows results from a study in patients. The median time between injection of the drug and the end of its pharmacological effect was 14.5 hours for NPH insulin while the median time for insulin glargine was 24 hours. The majority of patients on insulin glargine were still showing a response at this point of time, indicating an even longer duration of action. (See Figure.)
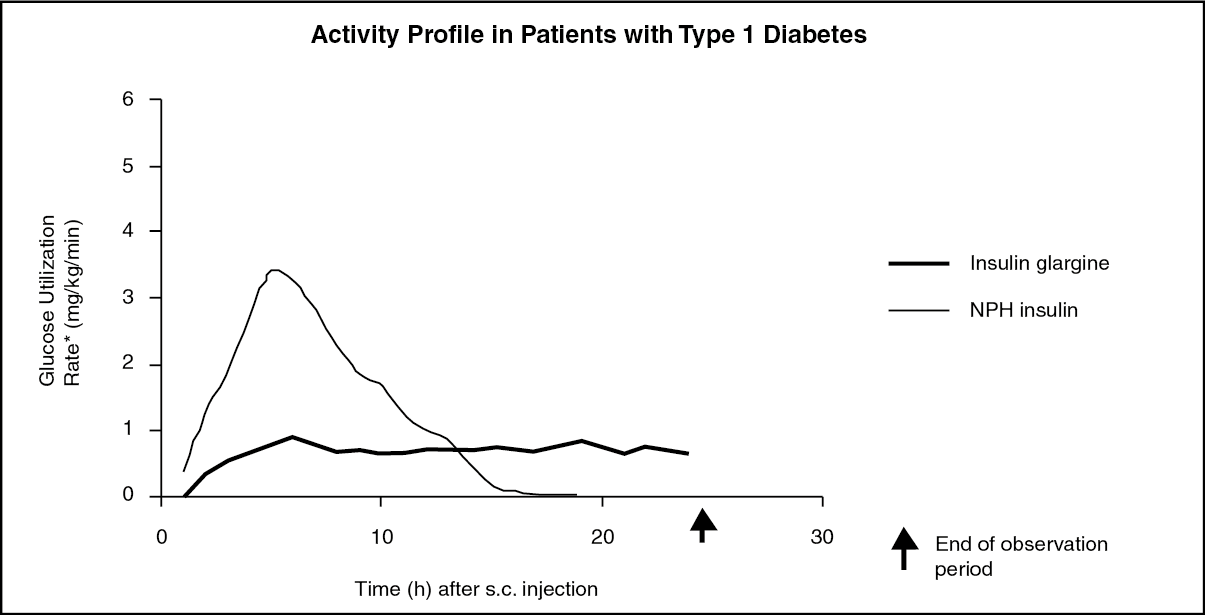
The longer duration of action of insulin glargine is directly related to its slower rate of absorption and supports once daily administration. The time course of action of insulin and insulin analogues such as insulin glargine may vary considerably in different individuals or within the same individual but is, due to the lack of a peak, less variable with insulin glargine than with NPH insulin.
An euglycaemic clamp study in healthy volunteers showed less intra-individual (day to day) variability in the pharmacodynamic profile for insulin glargine compared to ultralente human insulin.
Clinical Efficacy/Clinical Studies: The overall efficacy of once-daily insulin glargine on metabolic control was compared to that of once daily and twice-daily NPH human insulin in open-label, randomised, active-control, parallel studies of 2327 patients with type 1 diabetes mellitus and 1563 patients with type 2 diabetes mellitus. In general, insulin glargine maintained or improved the level of glycaemic control as measured by glycohemoglobin and fasting glucose. In addition, fewer patients using insulin glargine reported a hypoglycaemic episode compared to patients using NPH human insulin.
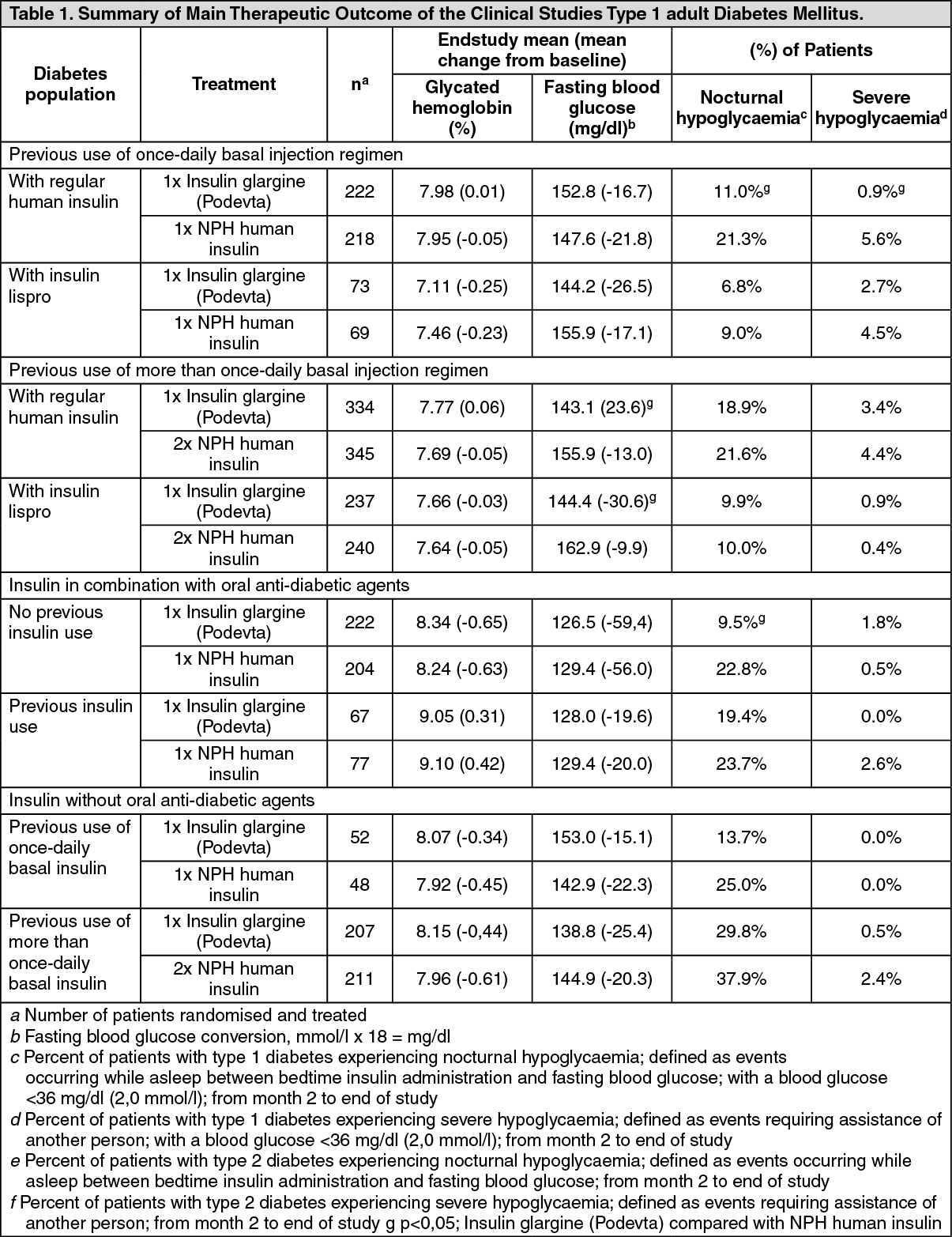
Type 1 Adult Diabetes (see Table 1): In Phase III studies, patients with type 1 diabetes (n=1119) were randomised to basal-bolus treatment with Insulin glargine (Podevta) once daily or to NPH human insulin once or twice daily and treated for 28 weeks. Regular human insulin was administered before each meal. Insulin glargine (Podevta) was administered at bedtime. NPH human insulin was administered once daily at bedtime or in the morning and at bedtime when used twice daily. Insulin glargine (Podevta) had a larger effect in reducing fasting glucose than NPH human insulin administered twice daily, but was comparable with NPH human insulin twice daily in its effect on glycohemoglobin and incidence of nocturnal and severe hypoglycaemia.
Compared to once daily NPH human insulin, Insulin glargine (Podevta) had a similar effect on fasting glucose and glycohemoglobin. However, fewer patients receiving Insulin glargine (Podevta) reported a severe hypoglycaemic episode after initial titration, from study month 2 onward, (0.9 % vs. 5.6 %, p<0.05) and fewer patients reported a nocturnal hypoglycaemic episode (11.0 % vs. 21.3 %, p<0,05).
Hypoglycaemia was reported with similar frequency during the first month of the studies after starting treatment with Insulin glargine (Podevta) compared to NPH human insulin.
In another Phase III study, patients with type 1 diabetes (n=619) were treated for 16 weeks with a basal bolus insulin regimen where insulin lispro was used before each meal. Insulin glargine (Podevta) was administered once daily at bedtime and NPH human insulin was administered once or twice daily.
Insulin glargine (Podevta) had a larger effect in reducing fasting glucose than NPH human insulin administered twice daily. Insulin glargine (Podevta) and NPH human insulin had a similar effect on glycohemoglobin, with similar numbers of patients reporting a hypoglycaemic episode.
Type 2 Diabetes (see Table 1): In one Phase III study (n=570), Insulin glargine (Podevta) was evaluated for 52 weeks as part of a regimen of combination therapy with insulin and oral anti-diabetic agents (a sulfonylurea, metformin, acarbose, or combinations of these drugs). Insulin glargine (Podevta) administered once daily at bedtime was as effective as NPH human insulin administered once daily at bedtime in reducing glycohemoglobin and fasting glucose.
However, fewer patients treated with Insulin glargine (Podevta) reported a nocturnal hypoglycaemic episode after initial titration, from study month 2 onward. This benefit of Insulin glargine (Podevta) was most pronounced in the subgroup of patients who had not previously been treated with insulin (Insulin glargine (Podevta): 9.5 %, NPH human insulin: 22.8 %; p<0.05).
In another Phase III study in patients with type 2 diabetes not using oral anti-diabetic agents (n=518), a basal-bolus regimen of Insulin glargine (Podevta) once daily at bedtime or NPH human insulin administered once or twice daily was evaluated for 28 weeks. Regular human insulin was used before meals as needed. Insulin glargine (Podevta) had similar effectiveness as either once or twice daily NPH human insulin in reducing glycohemoglobin and fasting glucose. However, fewer patients treated with Insulin glargine (Podevta) reported nocturnal hypoglycaemia from study month 2 onward than patients treated with NPH human insulin twice daily (29.8 % vs. 37.9 %, p=0.0582).
Type 1 Paediatric Diabetes (see Table 2): In a randomized, controlled clinical study, pediatric patients (age range 6 to 15 years) (study 3003) with type 1 diabetes (n=349) were treated for 28 weeks with a basal bolus insulin regimen where regular human insulin was used before each meal. Insulin glargine (Podevta) was administered once daily at bedtime and NPH human insulin was administered once or twice daily. Similar effects on glycohemoglobin and the incidence of hypoglycemia were observed in both treatment groups.
Type 1 Paediatric diabetes (1 to 6 years): A 24-week parallel group study was conducted in 125 children with type 1 diabetes mellitus aged 1 to 6 years (61 children from 2 to 5 in the insulin glargine group and 64 children from 1 to 6 in the NPH insulin group), comparing insulin glargine given once daily in the morning to NPH insulin given once or twice daily as basal insulin. Both groups received bolus insulin before meals.
Comparison of the two treatment regimens in terms of hypoglycemia was the primary objective of the study. The composite primary outcome consisted of: continuous glucose monitoring excursions below 70 mg/dL (3.9mM), confirmed by fingerstick blood glucose (FSBG) measurements; other FSBG measurements < 70 mg/dL; and episodes of symptomatic hypoglycemia.
Overall, the event rate ratio of this composite outcome for once daily Insulin glargine (Podevta) compared to NPH (given twice daily in most patients) was 1.18 (95 % CI: 0.97-1.44), therefore, not meeting the non-inferiority margin of 1.15.
The rate of symptomatic hypoglycemia events is the most commonly used and clinically relevant component of the composite outcome. Rates of symptomatic hypoglycemia events were numerically lower in the insulin glargine group, both overall (25.5 episodes per patient year, vs 33.0 for NPH) and overnight (2.38 episodes per patient-year, vs 3.65 for NPH).
Glycohaemoglobin and glucose variabilities were comparable in both treatment groups. No new safety signals were observed in this trial. (See Tables 1 and 2.)
An euglycaemic clamp study in healthy volunteers showed less intra-individual (day to day) variability in the pharmacodynamic profile for insulin glargine compared to ultralente human insulin.
Clinical Efficacy/Clinical Studies: The overall efficacy of once-daily insulin glargine on metabolic control was compared to that of once daily and twice-daily NPH human insulin in open-label, randomised, active-control, parallel studies of 2327 patients with type 1 diabetes mellitus and 1563 patients with type 2 diabetes mellitus. In general, insulin glargine maintained or improved the level of glycaemic control as measured by glycohemoglobin and fasting glucose. In addition, fewer patients using insulin glargine reported a hypoglycaemic episode compared to patients using NPH human insulin.
Type 1 Adult Diabetes (see Table 1): In Phase III studies, patients with type 1 diabetes (n=1119) were randomised to basal-bolus treatment with Insulin glargine (Podevta) once daily or to NPH human insulin once or twice daily and treated for 28 weeks. Regular human insulin was administered before each meal. Insulin glargine (Podevta) was administered at bedtime. NPH human insulin was administered once daily at bedtime or in the morning and at bedtime when used twice daily. Insulin glargine (Podevta) had a larger effect in reducing fasting glucose than NPH human insulin administered twice daily, but was comparable with NPH human insulin twice daily in its effect on glycohemoglobin and incidence of nocturnal and severe hypoglycaemia.
Compared to once daily NPH human insulin, Insulin glargine (Podevta) had a similar effect on fasting glucose and glycohemoglobin. However, fewer patients receiving Insulin glargine (Podevta) reported a severe hypoglycaemic episode after initial titration, from study month 2 onward, (0.9 % vs. 5.6 %, p<0.05) and fewer patients reported a nocturnal hypoglycaemic episode (11.0 % vs. 21.3 %, p<0,05).
Hypoglycaemia was reported with similar frequency during the first month of the studies after starting treatment with Insulin glargine (Podevta) compared to NPH human insulin.
In another Phase III study, patients with type 1 diabetes (n=619) were treated for 16 weeks with a basal bolus insulin regimen where insulin lispro was used before each meal. Insulin glargine (Podevta) was administered once daily at bedtime and NPH human insulin was administered once or twice daily.
Insulin glargine (Podevta) had a larger effect in reducing fasting glucose than NPH human insulin administered twice daily. Insulin glargine (Podevta) and NPH human insulin had a similar effect on glycohemoglobin, with similar numbers of patients reporting a hypoglycaemic episode.
Type 2 Diabetes (see Table 1): In one Phase III study (n=570), Insulin glargine (Podevta) was evaluated for 52 weeks as part of a regimen of combination therapy with insulin and oral anti-diabetic agents (a sulfonylurea, metformin, acarbose, or combinations of these drugs). Insulin glargine (Podevta) administered once daily at bedtime was as effective as NPH human insulin administered once daily at bedtime in reducing glycohemoglobin and fasting glucose.
However, fewer patients treated with Insulin glargine (Podevta) reported a nocturnal hypoglycaemic episode after initial titration, from study month 2 onward. This benefit of Insulin glargine (Podevta) was most pronounced in the subgroup of patients who had not previously been treated with insulin (Insulin glargine (Podevta): 9.5 %, NPH human insulin: 22.8 %; p<0.05).
In another Phase III study in patients with type 2 diabetes not using oral anti-diabetic agents (n=518), a basal-bolus regimen of Insulin glargine (Podevta) once daily at bedtime or NPH human insulin administered once or twice daily was evaluated for 28 weeks. Regular human insulin was used before meals as needed. Insulin glargine (Podevta) had similar effectiveness as either once or twice daily NPH human insulin in reducing glycohemoglobin and fasting glucose. However, fewer patients treated with Insulin glargine (Podevta) reported nocturnal hypoglycaemia from study month 2 onward than patients treated with NPH human insulin twice daily (29.8 % vs. 37.9 %, p=0.0582).
Type 1 Paediatric Diabetes (see Table 2): In a randomized, controlled clinical study, pediatric patients (age range 6 to 15 years) (study 3003) with type 1 diabetes (n=349) were treated for 28 weeks with a basal bolus insulin regimen where regular human insulin was used before each meal. Insulin glargine (Podevta) was administered once daily at bedtime and NPH human insulin was administered once or twice daily. Similar effects on glycohemoglobin and the incidence of hypoglycemia were observed in both treatment groups.
Type 1 Paediatric diabetes (1 to 6 years): A 24-week parallel group study was conducted in 125 children with type 1 diabetes mellitus aged 1 to 6 years (61 children from 2 to 5 in the insulin glargine group and 64 children from 1 to 6 in the NPH insulin group), comparing insulin glargine given once daily in the morning to NPH insulin given once or twice daily as basal insulin. Both groups received bolus insulin before meals.
Comparison of the two treatment regimens in terms of hypoglycemia was the primary objective of the study. The composite primary outcome consisted of: continuous glucose monitoring excursions below 70 mg/dL (3.9mM), confirmed by fingerstick blood glucose (FSBG) measurements; other FSBG measurements < 70 mg/dL; and episodes of symptomatic hypoglycemia.
Overall, the event rate ratio of this composite outcome for once daily Insulin glargine (Podevta) compared to NPH (given twice daily in most patients) was 1.18 (95 % CI: 0.97-1.44), therefore, not meeting the non-inferiority margin of 1.15.
The rate of symptomatic hypoglycemia events is the most commonly used and clinically relevant component of the composite outcome. Rates of symptomatic hypoglycemia events were numerically lower in the insulin glargine group, both overall (25.5 episodes per patient year, vs 33.0 for NPH) and overnight (2.38 episodes per patient-year, vs 3.65 for NPH).
Glycohaemoglobin and glucose variabilities were comparable in both treatment groups. No new safety signals were observed in this trial. (See Tables 1 and 2.)

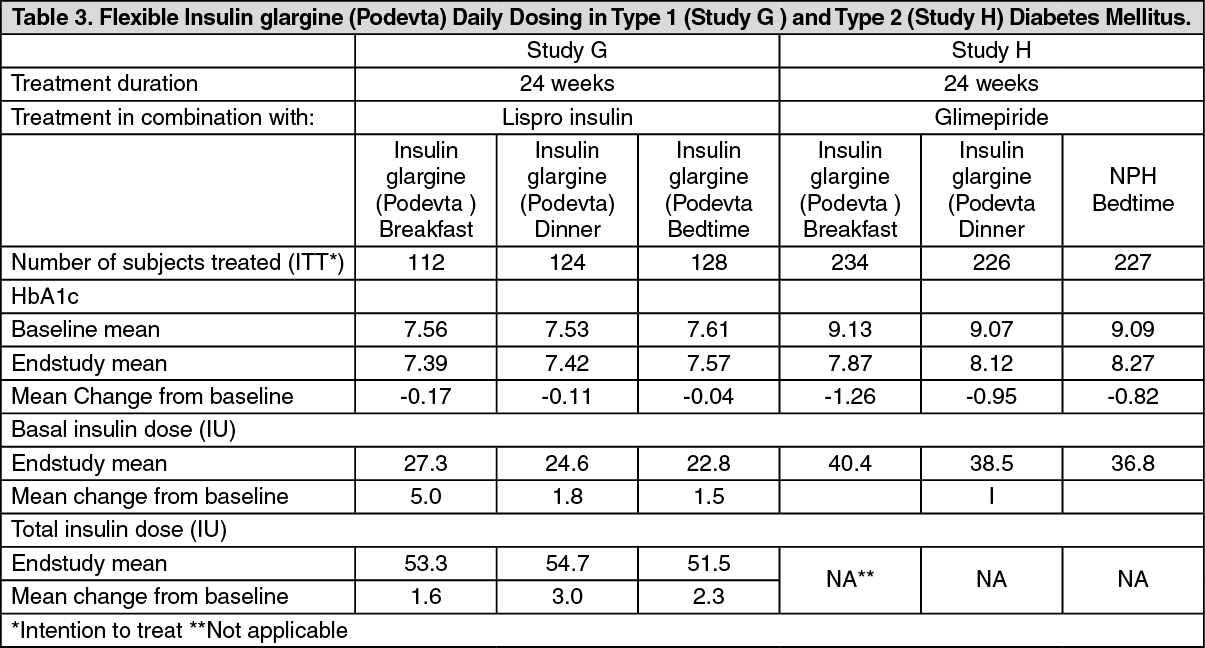
Flexible daily dosing: The safety and efficacy of Insulin glargine (Podevta) administered pre-breakfast, pre-dinner or at bedtime were evaluated in a large, randomized, controlled clinical study. In this study in patients with type 1 diabetes (Study G) (n=378), who were also treated with insulin lispro at meals, Insulin glargine (Podevta) administered at different times of the day resulted in equivalent glycemic control to that at bedtime. (See Table 3.)
The safety and efficacy of Insulin glargine (Podevta) administered pre-breakfast or at bedtime were also evaluated in a large, randomized, active-controlled clinical study (study H) (n=697) in type 2 diabetic patients no longer adequately controlled on oral agent therapy. All patients in this study also received glimepiride 3 mg daily.
Insulin glargine (Podevta) given before breakfast was at least as effective in lowering glycated hemoglobin A1c (HbA1c) as Insulin glargine (Podevta) given at bedtime or NPH human insulin given at bedtime. (See Table 3.)
The safety and efficacy of Insulin glargine (Podevta) administered pre-breakfast or at bedtime were also evaluated in a large, randomized, active-controlled clinical study (study H) (n=697) in type 2 diabetic patients no longer adequately controlled on oral agent therapy. All patients in this study also received glimepiride 3 mg daily.
Insulin glargine (Podevta) given before breakfast was at least as effective in lowering glycated hemoglobin A1c (HbA1c) as Insulin glargine (Podevta) given at bedtime or NPH human insulin given at bedtime. (See Table 3.)
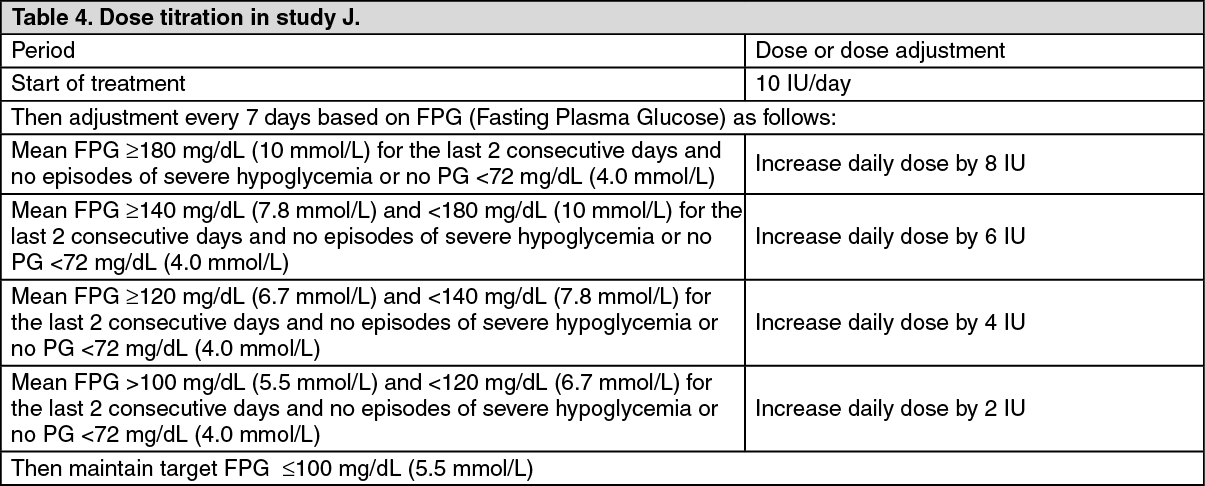
Type 2 Diabetes-Adult (Glycaemic control): In a randomized, open-label, parallel, 24-week clinical study (study J) in patients with type 2 diabetes (n=756) with an HbA1c > 7.5 % (mean 8.6 %) on one or two oral antidiabetes agents, Podevta or NPH insulin, once daily at bedtime, was added to their prior regimen. In order to reach the target fasting plasma glucose ≤100 mg/dL (5.5 mmol/L), the dose of Podevta and NPH was adjusted according to the structured dose-titration regimen as described in table 4. (See Table 4.)
Using this dose-titration schedule, HbA1c was reduced to a mean of 6.96 % for Podevta and 6.97 % for NPH insulin. More than half of the subjects in each group achieved a HbA1c value of 7.0 % (Podevta, 58.0 %; NPH insulin, 57.3 %; mean dose at study endpoint was 47.2 IU for Podevta and 41.8 IU for NPH).
In the Podevta-treated group, 33.2 % of the patients reached the primary efficacy endpoint (A1C value of 7.0 % in the absence of plasma glucose-confirmed nocturnal hypoglycemia ≤72 mg/dL [4 mmol/L]), compared to 26.7 % in the NPH-treated group (p= 0.0486).
Fewer patients treated with Podevta experienced nocturnal hypoglycemia compared with patients treated with NPH insulin. Other clinical trials in type 2 diabetes (study E, F and G) showed similar results (see also table 1) with less nocturnal hypoglycemia with patients treated Podevta compared to treated with NPH.
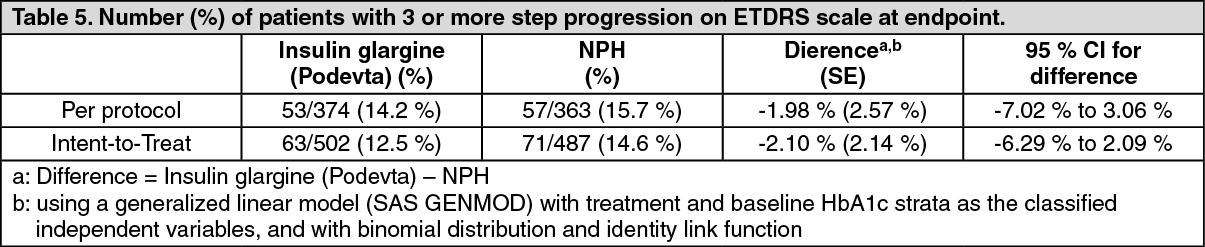
Diabetic Retinopathy: Effects of Insulin glargine (Podevta) on diabetic retinopathy were evaluated in a large 5-year NPH controlled study in which progression of retinopathy was investigated by fundus photography using a grading protocol derived from the Early Treatment Diabetic Retinopathy Study (ETDRS). The primary outcome in this study was progression by 3 or more steps on the ETDRS scale at study endpoint. The results of this analysis are shown in the table below for both the per-protocol (primary) and Intent to-Treat (ITT) populations, and indicate non inferiority of Insulin glargine (Podevta) to NPH in the progression of diabetic retinopathy as assessed by this outcome. (See Table 5.)
In the Podevta-treated group, 33.2 % of the patients reached the primary efficacy endpoint (A1C value of 7.0 % in the absence of plasma glucose-confirmed nocturnal hypoglycemia ≤72 mg/dL [4 mmol/L]), compared to 26.7 % in the NPH-treated group (p= 0.0486).
Fewer patients treated with Podevta experienced nocturnal hypoglycemia compared with patients treated with NPH insulin. Other clinical trials in type 2 diabetes (study E, F and G) showed similar results (see also table 1) with less nocturnal hypoglycemia with patients treated Podevta compared to treated with NPH.
Diabetic Retinopathy: Effects of Insulin glargine (Podevta) on diabetic retinopathy were evaluated in a large 5-year NPH controlled study in which progression of retinopathy was investigated by fundus photography using a grading protocol derived from the Early Treatment Diabetic Retinopathy Study (ETDRS). The primary outcome in this study was progression by 3 or more steps on the ETDRS scale at study endpoint. The results of this analysis are shown in the table below for both the per-protocol (primary) and Intent to-Treat (ITT) populations, and indicate non inferiority of Insulin glargine (Podevta) to NPH in the progression of diabetic retinopathy as assessed by this outcome. (See Table 5.)
Psychological outcomes: Patients with type 1 diabetes mellitus treated with regimens which included insulin glargine demonstrated significantly improved satisfaction with treatment when compared to patients on regimens with NPH insulin. (Diabetes Treatment Satisfaction Questionnaire).
ORIGIN Trial (Study 4032): The ORIGIN (Outcome Reduction with Initial Glargine Intervention) trial was a, international, multicenter, randomized, 2x2 factorial design study conducted in 12,537 participants with impaired fasting glucose (IFG), impaired glucose tolerance (IGT) or early type 2 diabetes mellitus and evidence of CV disease.
Participants were randomized to receive Insulin glargine (Podevta) (n=6264), titrated to a FPG of 95 mg/dL (5.3mM) or less, or Standard Care (n=6273). At baseline participants had a mean age of 63.5 years, mean duration of diabetes of 5.8 years in those with pre-existing diabetes, and median HbA1c of 6.4 %. Median duration of follow-up was approximately 6.2 years.
At the end of the trial 81 % of participants randomized to take Insulin glargine (Podevta) were still on treatment.
Median on-treatment HbA1c values ranged from 5.9 to 6.4 % in the Insulin glargine (Podevta) group, and 6.2 % to 6.6 % in the Standard Care group throughout the duration of follow-up.
Median FPG in the Insulin glargine (Podevta) group was at target (95 mg/dL) following dose titration for the duration of the study.
The rates of severe hypoglycemia (affected participants per 100 participant years of exposure) were 1.05 for insulin glargine and 0.30 for Standard Care group. Overall, severe hypoglycemia was reported for 3.7 % of these participants over the course of this 6 year study (approximately 0.6 % per participant-year).
The median of the change in body weight from baseline to the last on-treatment visit was 2.2 kg greater in the Podevta group than in the Standard Care group.
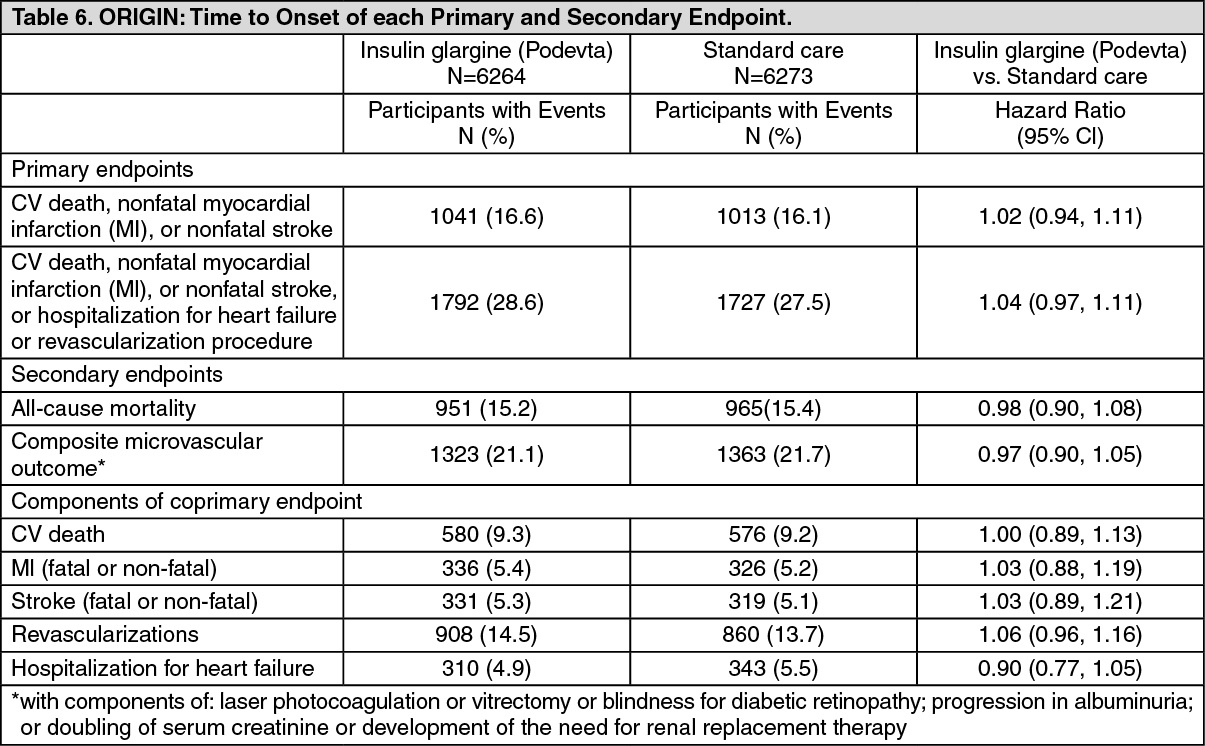
The primary objective of this trial was to examine the effect of Insulin glargine (Podevta) on two coprimary composite efficacy outcomes. The first one was the time to the first occurrence of CV death, nonfatal myocardial infarction (MI), or nonfatal stroke, and the second one was the time to the first occurrence of any of the first co-primary events, or revascularization procedure (cardiac, carotid, or peripheral), or hospitalization for heart failure.
Secondary endpoints were: all-cause mortality; a composite microvascular outcome; development of type 2 diabetes, in participants with IGT and/or IFG at baseline.
The primary and secondary outcome results, as well as the results for each component of the coprimary outcomes, are displayed in the two tables (Table 6 for the time-to-event analyses, and, for the non-time-to-event analysis of development of diabetes, Table 7) as follows. (See Tables 6 and 7.)
ORIGIN Trial (Study 4032): The ORIGIN (Outcome Reduction with Initial Glargine Intervention) trial was a, international, multicenter, randomized, 2x2 factorial design study conducted in 12,537 participants with impaired fasting glucose (IFG), impaired glucose tolerance (IGT) or early type 2 diabetes mellitus and evidence of CV disease.
Participants were randomized to receive Insulin glargine (Podevta) (n=6264), titrated to a FPG of 95 mg/dL (5.3mM) or less, or Standard Care (n=6273). At baseline participants had a mean age of 63.5 years, mean duration of diabetes of 5.8 years in those with pre-existing diabetes, and median HbA1c of 6.4 %. Median duration of follow-up was approximately 6.2 years.
At the end of the trial 81 % of participants randomized to take Insulin glargine (Podevta) were still on treatment.
Median on-treatment HbA1c values ranged from 5.9 to 6.4 % in the Insulin glargine (Podevta) group, and 6.2 % to 6.6 % in the Standard Care group throughout the duration of follow-up.
Median FPG in the Insulin glargine (Podevta) group was at target (95 mg/dL) following dose titration for the duration of the study.
The rates of severe hypoglycemia (affected participants per 100 participant years of exposure) were 1.05 for insulin glargine and 0.30 for Standard Care group. Overall, severe hypoglycemia was reported for 3.7 % of these participants over the course of this 6 year study (approximately 0.6 % per participant-year).
The median of the change in body weight from baseline to the last on-treatment visit was 2.2 kg greater in the Podevta group than in the Standard Care group.
The primary objective of this trial was to examine the effect of Insulin glargine (Podevta) on two coprimary composite efficacy outcomes. The first one was the time to the first occurrence of CV death, nonfatal myocardial infarction (MI), or nonfatal stroke, and the second one was the time to the first occurrence of any of the first co-primary events, or revascularization procedure (cardiac, carotid, or peripheral), or hospitalization for heart failure.
Secondary endpoints were: all-cause mortality; a composite microvascular outcome; development of type 2 diabetes, in participants with IGT and/or IFG at baseline.
The primary and secondary outcome results, as well as the results for each component of the coprimary outcomes, are displayed in the two tables (Table 6 for the time-to-event analyses, and, for the non-time-to-event analysis of development of diabetes, Table 7) as follows. (See Tables 6 and 7.)

There were no statistical significant differences between treatment groups in the overall incidence of cancer (all types combined) or death from cancer. The time to first event of any cancer or new cancer during the study was similar between the two treatment groups with respective hazard ratios of 0.99 (0.88, 1.11) and 0.96 (0.85, 1.09).
Participation in ORIGIN for a median of approximately 6.2 years showed that treatment with Podevta did not alter the risk for cardiovascular outcomes, all-cause mortality or cancer, when compared to standard glucose lowering therapy. In addition, metabolic control was maintained at a lower level of glycemia, with a decrease in the percentage of participants developing diabetes, at a cost of a modest increase in hypoglycemia and weight gain.
Pharmacokinetics: After subcutaneous injection of insulin glargine in healthy subjects and diabetic patients, the insulin serum concentrations indicated a slower and much more prolonged absorption and a lack of a peak in comparison to human NPH insulin. Concentrations were, thus, consistent with the time profile of the pharmacodynamic activity of insulin glargine.
After subcutaneous injection of 0.3 IU/kg insulin glargine in diabetic patients, a flat concentration-time profile has been demonstrated; this is also reflected in the wide range of tmax values (between 1.5 and 22.5 hours) compared to NPH (2.5 to 10.0 hours).
When given intravenously, the concentration profiles and the apparent elimination half-life of insulin glargine and human insulin were comparable.
There were no relevant differences in serum insulin levels after abdominal, deltoid or thigh administration of insulin glargine.
Insulin glargine has less intra- and inter-individual variability in pharmacokinetic profile compared to human ultralente insulin.
After subcutaneous injection of Insulin glargine (Podevta) in healthy subjects and diabetic patients, insulin glargine is rapidly metabolized at the carboxyl terminus of the Beta chain with formation of two active metabolites M1 (21AGly-insulin) and M2 (21A-Gly-des-30B-Thrinsulin). In plasma, the principal circulating compound is the metabolite M1. The exposure to M1 increases with the administered dose of Insulin glargine (Podevta). The pharmacokinetic and pharmacodynamic findings indicate that the effect of the subcutaneous injection with Insulin glargine (Podevta) is principally based on exposure to M1. Insulin glargine and the metabolite M2 were not detectable in the vast majority of subjects and, when they were detectable their concentration was independent of the administered dose of Insulin glargine (Podevta).
Special populations: Age and Gender: Information on the effect of age and gender on the pharmacokinetics of insulin glargine is unavailable. However, in large clinical trials, subgroup analysis based on age and gender did not indicate any difference in safety and efficacy in insulin glargine treated patients over the entire study population. The same holds true for NPH treated patients.
Smoking: In clinical trials a subgroup analysis showed no differences in safety and efficacy of insulin glargine between the group of smokers and the total study population. The same is true for NPH insulin.
Obesity: In clinical trials subgroup analysis based on BMI showed no differences in safety and efficacy of insulin glargine in this group of patients compared to the total study population.
The same is true for NPH insulin.
Children: Pharmacokinetics in children aged 2 to less than 6 years of age with type 1 diabetes mellitus was assessed in one clinical study (see Pharmacodynamics previously). Plasma "trough" levels of insulin glargine and its main metabolites M1 and M2 were measured in children treated with insulin glargine, revealing plasma concentration patterns similar to adults, and providing no evidence for accumulation of insulin glargine or its metabolites with chronic dosing.
Toxicology: Non-Clinical Safety Data: Acute Toxicity: The acute toxicity of intravenous and subcutaneous administration of insulin glargine was tested in mice and rats. The LD50 in each species was in the range of 1000 IU/kg.
Chronic Toxicity: In repeated subcutaneous dose toxicity studies of insulin glargine in mice, rats and dogs only expected pharmacodynamic effects were observed.
Carcinogenicity: Two-year carcinogenicity studies were performed in rats and mice. The results do not indicate a risk to humans.
Genotoxicity: Insulin glargine was not mutagenic in tests for detection of gene mutations in bacteria and mammalian cells (Ames- and HGPRT-test) and in tests for detection of chromosomal aberrations (Cytogenetics in vitro in V79-cells and in vivo in Chinese hamsters).
Impairment of Fertility: Reproduction toxicity: In an embryotoxicity study in rats, hypoglycaemia but no maternal toxicity occurred. Insulin glargine was not embryotoxic and not teratogenic.
In an embryotoxicity study in rabbits, maternal (hypoglycaemic shock, intrauterine deaths) and embryofoetal toxicity, due to hypoglycaemia, was observed, including single anomalies in the middle and high-dose groups. Similar effects were obtained with an intermediate acting marketed insulin.
In a combined fertility and pre- and postnatal study in rats, maternal toxicity due to dose dependent hypoglycaemia was observed. Some deaths, and consequently a reduction of the rearing rate, occurred in the high-dose group only. Similar effects were obtained with an intermediate acting marketed insulin.
Local tolerance: Local tolerability studies with subcutaneous, intramuscular, intravenous and paravenous administration in rabbits gave no indication of risk for the use of insulin glargine in man.
Immunogenicity: Standard immunogenicity studies performed in pigs, rabbits and guinea pigs indicated a similar or lower immunogenic potential for insulin glargine than for human insulin in these species.
Participation in ORIGIN for a median of approximately 6.2 years showed that treatment with Podevta did not alter the risk for cardiovascular outcomes, all-cause mortality or cancer, when compared to standard glucose lowering therapy. In addition, metabolic control was maintained at a lower level of glycemia, with a decrease in the percentage of participants developing diabetes, at a cost of a modest increase in hypoglycemia and weight gain.
Pharmacokinetics: After subcutaneous injection of insulin glargine in healthy subjects and diabetic patients, the insulin serum concentrations indicated a slower and much more prolonged absorption and a lack of a peak in comparison to human NPH insulin. Concentrations were, thus, consistent with the time profile of the pharmacodynamic activity of insulin glargine.
After subcutaneous injection of 0.3 IU/kg insulin glargine in diabetic patients, a flat concentration-time profile has been demonstrated; this is also reflected in the wide range of tmax values (between 1.5 and 22.5 hours) compared to NPH (2.5 to 10.0 hours).
When given intravenously, the concentration profiles and the apparent elimination half-life of insulin glargine and human insulin were comparable.
There were no relevant differences in serum insulin levels after abdominal, deltoid or thigh administration of insulin glargine.
Insulin glargine has less intra- and inter-individual variability in pharmacokinetic profile compared to human ultralente insulin.
After subcutaneous injection of Insulin glargine (Podevta) in healthy subjects and diabetic patients, insulin glargine is rapidly metabolized at the carboxyl terminus of the Beta chain with formation of two active metabolites M1 (21AGly-insulin) and M2 (21A-Gly-des-30B-Thrinsulin). In plasma, the principal circulating compound is the metabolite M1. The exposure to M1 increases with the administered dose of Insulin glargine (Podevta). The pharmacokinetic and pharmacodynamic findings indicate that the effect of the subcutaneous injection with Insulin glargine (Podevta) is principally based on exposure to M1. Insulin glargine and the metabolite M2 were not detectable in the vast majority of subjects and, when they were detectable their concentration was independent of the administered dose of Insulin glargine (Podevta).
Special populations: Age and Gender: Information on the effect of age and gender on the pharmacokinetics of insulin glargine is unavailable. However, in large clinical trials, subgroup analysis based on age and gender did not indicate any difference in safety and efficacy in insulin glargine treated patients over the entire study population. The same holds true for NPH treated patients.
Smoking: In clinical trials a subgroup analysis showed no differences in safety and efficacy of insulin glargine between the group of smokers and the total study population. The same is true for NPH insulin.
Obesity: In clinical trials subgroup analysis based on BMI showed no differences in safety and efficacy of insulin glargine in this group of patients compared to the total study population.
The same is true for NPH insulin.
Children: Pharmacokinetics in children aged 2 to less than 6 years of age with type 1 diabetes mellitus was assessed in one clinical study (see Pharmacodynamics previously). Plasma "trough" levels of insulin glargine and its main metabolites M1 and M2 were measured in children treated with insulin glargine, revealing plasma concentration patterns similar to adults, and providing no evidence for accumulation of insulin glargine or its metabolites with chronic dosing.
Toxicology: Non-Clinical Safety Data: Acute Toxicity: The acute toxicity of intravenous and subcutaneous administration of insulin glargine was tested in mice and rats. The LD50 in each species was in the range of 1000 IU/kg.
Chronic Toxicity: In repeated subcutaneous dose toxicity studies of insulin glargine in mice, rats and dogs only expected pharmacodynamic effects were observed.
Carcinogenicity: Two-year carcinogenicity studies were performed in rats and mice. The results do not indicate a risk to humans.
Genotoxicity: Insulin glargine was not mutagenic in tests for detection of gene mutations in bacteria and mammalian cells (Ames- and HGPRT-test) and in tests for detection of chromosomal aberrations (Cytogenetics in vitro in V79-cells and in vivo in Chinese hamsters).
Impairment of Fertility: Reproduction toxicity: In an embryotoxicity study in rats, hypoglycaemia but no maternal toxicity occurred. Insulin glargine was not embryotoxic and not teratogenic.
In an embryotoxicity study in rabbits, maternal (hypoglycaemic shock, intrauterine deaths) and embryofoetal toxicity, due to hypoglycaemia, was observed, including single anomalies in the middle and high-dose groups. Similar effects were obtained with an intermediate acting marketed insulin.
In a combined fertility and pre- and postnatal study in rats, maternal toxicity due to dose dependent hypoglycaemia was observed. Some deaths, and consequently a reduction of the rearing rate, occurred in the high-dose group only. Similar effects were obtained with an intermediate acting marketed insulin.
Local tolerance: Local tolerability studies with subcutaneous, intramuscular, intravenous and paravenous administration in rabbits gave no indication of risk for the use of insulin glargine in man.
Immunogenicity: Standard immunogenicity studies performed in pigs, rabbits and guinea pigs indicated a similar or lower immunogenic potential for insulin glargine than for human insulin in these species.
MedsGo Class
Insulin Preparations
Features
Dosage
100 I.U. / ml (3.64 mg Insulin glargine equivalent to 100 units Human insulin)
Ingredients
- Insulin Glargine
Packaging
Solution for Injection (S.C.) 3 ml x 5's
Generic Name
Insulin Glargine
Registration Number
BRP-013-01
Classification
Prescription Drug (RX)
Reviews
No reviews found
Product Questions
Questions


